1323
Automated Contrast Selection for Robust Bright- and Black-Blood Myocardial Scar Imaging1IHU LIRYC, Electrophysiology and Heart Modeling Institute, Université de Bordeaux – INSERM U1045, Avenue du Haut Lévêque, 33604, Pessac, France, Bordeaux, France, 2Department of Diagnostic and Interventional Radiology, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland, Lausanne, Switzerland, 3Department of Cardiovascular Imaging, Hôpital Cardiologique du Haut-Lévêque, CHU de Bordeaux, Avenue de Magellan, 33604 Pessac, France, Bordeaux, France, 4CIBM Center for Biomedical Imaging, Lausanne, Switzerland, Lausanne, Switzerland
Synopsis
Keywords: Heart, Data Processing, late gadolinium enhancement, black-blood, TI scout
Phase-sensitive inversion recovery is the reference imaging technique for the assessment of myocardial scars. Despite its ability to provide excellent contrast between healthy and scar tissue, small subendocardial scars can be challenging to detect due to poor scar-to-blood contrast. Joint bright- and black-blood late gadolinium enhancement techniques have been developed to provide both scar and anatomy information. Black-blood contrast is obtained after manual selection of an optimal inversion time (TI). This often results in uncertainties, variability, increased workload, and operator-dependency. In this work, we propose a method exploiting artificial intelligence to fully automate TI selection for more robust cardiac imaging.Introduction
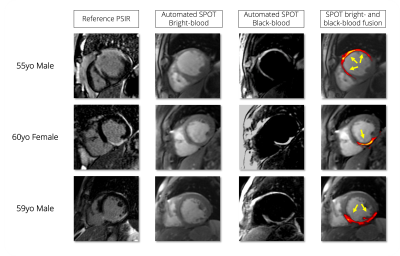
Phase-sensitive inversion recovery (PSIR) is the reference imaging technique for the assessment of myocardial scars1. Despite the excellent contrast between healthy and scar tissue, detection of small subendocardial scars is often hampered by the poor contrast at the blood-scar interface on PSIR imaging. Joint bright- and black-blood late gadolinium enhancement (LGE) techniques2 have thus been developed to provide images with both improved scar contrast (from black-blood images) and improved scar localization (from bright-blood images). Black-blood contrast is achieved by the visual selection of an optimal inversion time (TI) on a prior TI scout sequence. The corresponding TI image should present the darkest blood and the darkest healthy myocardium signals to reach the ideal scar-to-blood and scar-to-healthy myocardium contrasts. This manual, operator-dependant, selection often results in uncertainties, inter- and intra-expert variability, and increased workload.Here, we propose a method exploiting artificial intelligence (AI) to fully automate TI selection and simplify joint bright- and black-blood LGE imaging.
Methods
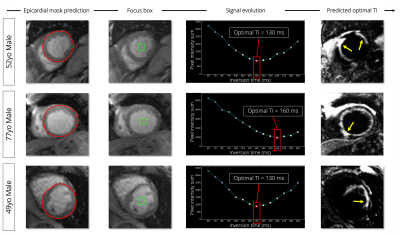
Image acquisition:Short-axis 2D images were collected on a 1.5-T system (Siemens, MAGNETOM Aera) using the free-breathing single-shot SPOT sequence (Figure 1). Image resolution was 1.5x1.5mm2 (8mm slice thickness). A dedicated TI scout was performed prior to SPOT to collect black-blood images with increasing TIs in the odd heartbeats and bright-blood images in the even heartbeats. TI scout values ranged from 60ms to 190ms with a 10ms increment. The optimal TI corresponds to the TI image with the darkest blood and the darkest healthy myocardium signals. The goal is to automatically find this image and retrieve its corresponding TI value (“optimal TI”). The algorithm is organised in three steps detailed hereafter (Figure 2).
Image processing:
Step 1: A fully automated spatial localization of the left ventricle is performed. To that end, a U-Net3 architecture is proposed to segment the epicardium on the first bright-blood image collected. The U-Net architecture was trained with 186 patients (~2400 images) with the following hyper parameters: learning rate = 10-3, batch size = 32, epochs = 200, binary cross-entropy loss, Adam optimizer. Whole-heart epicardium labels were manually drawn by expert operators for each patient.
Step 2: The centre of gravity of the predicted epicardium contour is computed to create a square region-of-interest centred on the resulting pixel (“focus box”), that is then propagated on all TI-varying black-blood images.
Step 3: The sum of pixel intensities inside these focus boxes is computed for each black-blood image with inversion recovery. The lowest sum corresponds to the image with the darkest blood and darkest healthy myocardium signals and the corresponding TI is considered as optimal.
The algorithm was implemented inline in our MRI systems using the Gadgetron framework4 for routine clinical use.
Experiments:
Our proposed algorithm was validated on a dataset of 152 patients, never seen by the U-Net, with known ischemic heart disease. Optimal TI values were measured for each patient with the proposed fully-automated algorithm. Thirty focus box sizes, ranging from 2.3mm2 to 20.3cm2, were tested. Two expert operators provided optimal TI values for each patient and a ground truth consensus was obtained for all data. One expert provided two sets of optimal TI values in two separated sessions.
The agreement between the predicted TI and the ground truth consensus was derived. Inter- and intra-expert agreements were calculated. Processing times were measured.
Results
The proposed algorithm extracted the TI value in 2.9s per patient (vs. ~20s for the experts). The algorithm reached an agreement of 91.5% (139/152 patients) with a focus box size of 1.8cm2 (9 x 9 pixels), assuming that a 10ms difference between the algorithm’s prediction and the ground truth consensus is considered acceptable. The agreement was always above 88% for a focus box size between 1.1cm2 and 10.6cm2.In the case where only one ground truth TI value (consensus) is considered per patient, we observed a 47.4% agreement between the ground truth consensus and the predicted TI values (Figure 3). Inter- and intra-expert agreements in TI selection were 61.2% and 67.8%, respectively.
Examples of optimal TI predictions are shown in Figure 4 and Figure 5 for six patients with myocardial infarction.
Conclusion
The proposed algorithm accurately predicted the optimal TI values of joint bright- and black-blood LGE imaging in a fast and fully automated way, thus reducing uncertainties, operator-dependency, and workload inherent to manual TI selection while removing the effects of inter- and intra-expert variability. These results are promising for future clinical use.Acknowledgements
This research was supported by funding from the French National Research Agency under grant agreements Equipex MUSIC ANR-11-EQPX-0030, ANR-21-CE17-0034-01, Programme d’Investissements d’Avenir ANR-10-IAHU04-LIRYC, and ANR Chaire Professeur Junior and from the European Council under grant agreement ERC n715093. A.B. acknowledges a Lefoulon-Delalande Foundation fellowship administered by the Institute of France.References
1. Kellman P, Arai AE, McVeigh ER, Aletras AH. Phase-sensitive inversion recovery for detecting myocardial infarction using gadolinium-delayed hyperenhancement. Magnetic resonance in medicine. 2002;47(2):372-383. doi:10.1002/mrm.10051
2. Bustin A, Sridi S, Maillot A, et al. Improved myocardial scar visualization with two-minute free-breathing joint bright- and black-blood late gadolinium enhancement imaging. In: Proceedings from the Joint Annual Meeting ISMRM-ESMRMB. ; 2022:0271.
3. Ronneberger O, Fischer P, Brox T. U-Net: Convolutional Networks for Biomedical Image Segmentation. Medical Image Computing and Computer-Assisted Intervention. Published online 2015:234-241. doi:10.1007/978-3-319-24574-4_28
4. Hansen MS, Sørensen TS. Gadgetron: an open source framework for medical image reconstruction. Magnetic resonance in medicine. 2013;69(6):1768-1776. doi:10.1002/mrm.24389
Figures
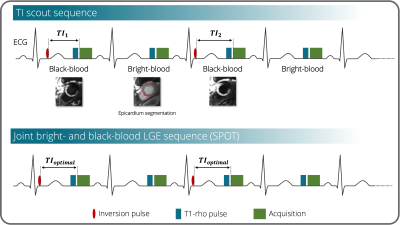
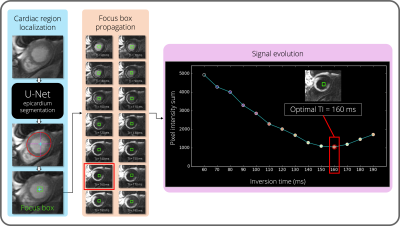
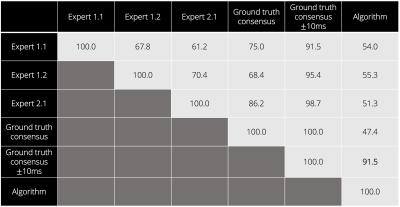
Figure 3. Agreement table (in %) between the expert operators (Expert 1, Expert 2) and the algorithm TI prediction, and between the ground truth consensus and the algorithm TI prediction. Manual expert selections are annotated as Expert X.Y for Expert X session Y.