1316
Highly-Efficient 3D free-breathing whole-heart MRA in 3 min: Clinical validation in patients with adult congenital heart disease1King's College London, London, United Kingdom, 2St Thomas’ Hospital, Guy’s and St Thomas’ NHS Foundation Trust, London, United Kingdom, 3MR Research Collaborations, Siemens Healthcare Limited, Camberley, United Kingdom, 4School of Engineering, Pontificia Universidad Católica de Chile, Santiago, Chile, 5Institute for Biological and Medical Engineering, Pontificia Universidad Católica de Chile, Santiago, Chile, 6Millennium Institute for Intelligent Healthcare Engineering, Santiago, Chile
Synopsis
Keywords: Vessels, Cardiovascular, angiography
Cardiovascular MRA is established for serial anatomical evaluation of patients with congenital heart disease (CHD). However, this approach is limited by diaphragmatic respiratory navigation, that leads to long acquisition times and degraded image quality due to residual motion artefacts. Here we evaluate a novel accelerated whole-heart framework in patients with adult CHD. This approach incorporates image-based navigation for translational and non-rigid motion-correction along with 3D patch-based denoising for efficient, free-breathing 3D whole-heart imaging. Comparison between the conventional and the research sequence shows superior diagnostic confidence and diagnostic accuracy for the proposed technique in significantly faster acquisition time (~3min proposed,~15min clinical).Introduction
Three-dimensional, whole heart, T2-prepared, balanced steady state free precession (T2prep-bSSFP) sequences can delineate the intra-cardiac and vascular anatomy and are established for anatomical assessment in patients with congenital heart disease (CHD)1. However, they are limited by long acquisition times, due to their conventional reliance on diaphragmatic respiratory gating (dNAV T2prep-bSSFP) to minimize the effects of respiratory motion, as well as residual motion artefacts. A recently proposed accelerated cardiovascular MRA framework, which incorporates image-based navigation2,3 for translational and non-rigid motion correction, along with 3D patch-based denoising (iNAV T2prep-bSSFP PROST)4, was prospectively evaluated in this study against the current clinical sequence in adult patients with CHD.Methods
Forty adult patients with CHD and different underlying pathologies (tetralogy of Fallot, coarctation of the aorta, hypoplastic left heart syndrome, atrioventricular septal defect, transposition of the great arteries, anomalous pulmonary venous drainage) were scanned on a 1.5T system (MAGNETOM Aera, Siemens Healthcare). The proposed ECG-triggered, free-breathing research sequence was acquired with a 4-fold undersampled variable-density Cartesian trajectory5 (FOV: 400x300x72-108 mm3, resolution 1.5 mm3, flip angle =90°, T2-prep duration = 40ms, TE/TR = 1.75/238 ms, coronal orientation). 2D image-based navigators2 were acquired at each cardiac cycle by spatially encoding the start-up echoes preceding the 3D CMRA acquisition to enable 100% respiratory scan efficiency. Motion estimation and non-rigid motion correction was performed inline in the scanner software. 3D-PROST denoising was performed off-line (Fig. 1). The research sequence was validated against the clinical sequence with matching parameters (except FOV 400x300x88-120, TE/TR = 1.52/294 ms, GRAPPA parallel imaging 2x undersampled, sagittal orientation). Signal ratio for all the major intrapericardiac structures was computed for the datasets of both techniques. Two blinded experts reviewed the research and the clinical datasets in a completely randomized order and recorded their diagnostic confidence for full anatomical analysis (1: poor diagnostic confidence to 4: full diagnostic confidence). Furthermore, each clinician was asked to identify the presence of the following abnormalities: 1) Main Pulmonary Artery stenosis/dilatation, 2) Right pulmonary artery stenosis/dilatation, 3) left pulmonary artery stenosis/dilatation, 4) coronary artery abnormalities, 5) aortic dilatation/aneurysm, 6) aortic arch abnormalities, 7) anomalous pulmonary venous connections. Each abnormality was scored on a 5-point Likert scale (1 = Definitely not present, 2 = Probably not present, 3 = Unclear, 4 = Probably present, 5 = Definitely present). For the evaluation of diagnostic accuracy scores of 1 and 2 were coded as abnormality is absent, and 4 and 5 were coded as abnormality is present. A score of 3 was coded as a misdiagnosis. Quantitative (signal ratio) and qualitative image scores (diagnostic accuracy and diagnostic confidence) were compared between the two approaches.Results
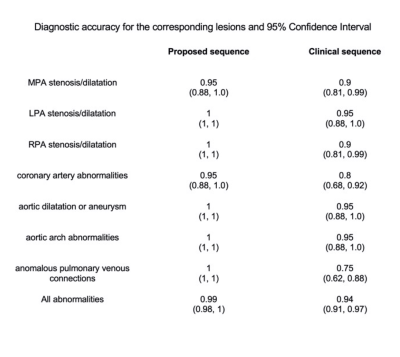
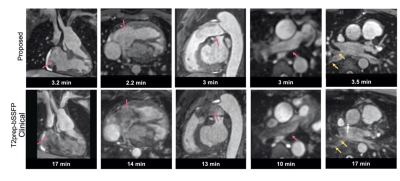
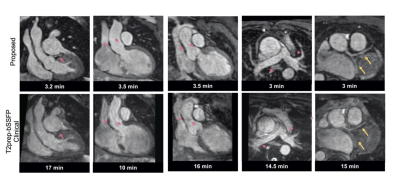
The proposed technique achieved significantly shorter acquisition time [3.2±0.7min (proposed) versus 15±3.2min (clinical), P<0.0001] (Fig. 2A). Reconstruction time for the proposed approach was 2±0.5min (inline) plus 1.5±0.5min (offline). Signal ratio comparison demonstrated comparable results with both methods for all structures, except the pulmonary veins where the proposed approach was superior (Fig. 2B). The proposed approach demonstrated a significant increase in diagnostic confidence for both reviewers [Reviewer 1: 4(3.5, 4) vs 3(2.5, 3), P=0.007; Reviewer 2: 4(4, 4) vs 4 (3, 4), P=0.009]. The overall diagnostic accuracy for the diagnosis of main and branch pulmonary artery abnormalities, aortic aneurysm/dilatation, aortic arch abnormalities, coronary artery abnormalities and anomalous pulmonary venous connections was statistically significant higher for the proposed sequence versus the clinical (0.99 vs 0.94, P<0.001) (Fig. 3). Furthermore, visual comparison of the images from both techniques demonstrated that flow and off resonance artefacts along with respiratory and non-rigid motion artefacts, were attenuated with the proposed approach (Fig. 4 & 5).Discussion
The proposed free-breathing whole-heart MRA with image-based navigation, non-rigid motion compensated reconstruction and PROST denoising framework demonstrated high quality depiction of the heart and the thoracic vessels in adult patients with CHD, in shorter and more consistent scan times (~ 3min) than the current clinical standard.Conclusion
Future validation in multi-center studies is warranted to facilitate clinical adoption.Acknowledgements
The authors acknowledge financial support from the BHF PG/18/59/33955, EPSRC EP/P001009, EP/P032311/1, EP/P007619/1, EP/V044087/1, Wellcome EPSRC Centre for Medical Engineering (NS/A000049/1), Millennium Institute for Intelligent Healthcare Engineering ICN2021_004, Fondecyt 1210637, Fondecyt 1210638, ANID Basal FB210024, Millennium Nucleus NCN19_161.References
1. Fratz S, Chung T, Greil GF, et al. Guidelines and protocols for cardiovascular magnetic resonance in children and adults with congenital heart disease: SCMR expert consensus group on congenital heart disease. J Cardiovasc Magn Reson. 2013;15(1):51. Published 2013 Jun 13. doi:10.1186/1532-429X-15-51.
2. Henningsson M, Smink J, Razavi R, Botnar RM. Prospective respiratory motion correction for coronary MR angiography using a 2D image navigator. Magn Reson Med. 2013;69(2):486-494. doi:10.1002/mrm.24280.
3. Cruz G, Atkinson D, Henningsson M, Botnar RM, Prieto C. Highly efficient nonrigid motion-corrected 3D whole-heart coronary vessel wall imaging. Magn Reson Med. 2017;77(5):1894-1908. doi:10.1002/mrm.26274.
4. Bustin A, Lima da Cruz G, Jaubert O, Lopez K, Botnar RM, Prieto C. High-dimensionality undersampled patch-based reconstruction (HD-PROST) for accelerated multi-contrast MRI. Magn Reson Med. 2019;81(6):3705-3719. doi:10.1002/mrm.27694.
5. Prieto C, Doneva M, Usman M, et al. Highly efficient respiratory motion compensated free-breathing coronary MRA using golden-step Cartesian acquisition. J Magn Reson Imaging. 2015;41(3):738-746. doi:10.1002/jmri.24602.
Figures
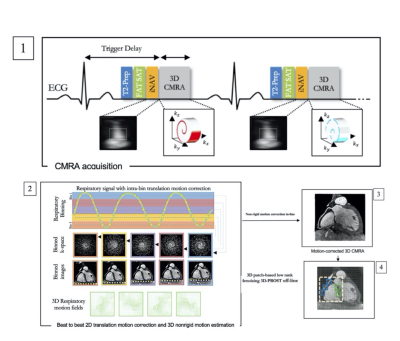
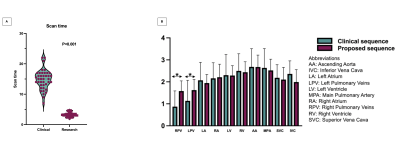
Figure 2A: Scan time is more consistent and statistically significantly shorter with the proposed sequence.
Figure 2B: Signal ratio comparison for the major intrapericardiac structures between the proposed and the clinical sequence. Asterisks (*) indicate P<0.05