1312
Aging-related morphology and microstructure variations: Discoveries from the Lifespan Human Connectome Project - Aging1Rotman Research Institute, Baycrest, Toronto, ON, Canada, 2Medical Biophysics, University of Toronto, Toronto, ON, Canada, 3Biomedical Engineering, University of Toronto, Toronto, ON, Canada
Synopsis
Keywords: White Matter, Microstructure
This study compared age-related differences in white matter morphology and microstructure across ten major tracts of the human brain using diffusion data from 535 participants of the Human Connectome Project in Aging. The results are additionally assessed for agreement with retrogenesis predictions of white matter decline in normal aging. While whole-brain relationships between morphometry and white matter integrity were identified, high variability was also observed between tracts. While our data do not fully support retrogenesis models, we demonstrate patterns that may provide partial support, and highlight the need for tract-specific studies of morphological-microstructural interactions in the aging white matter.Introduction
Progressive age-related changes in white matter (WM) morphometry and microstructure have both been long been observed1,2,5. The ability to predict these changes across WM tracts and build an integrated model of age-related WM trajectories is valuable for developing compensatory strategies for aging adults. However, there has been relatively little exploration into other attributes of tract morphology or its relation to microstructural measures in vivo. Using diffusion MRI data from the Human Connectome Project in Aging (HCP-A)3, this study seeks to examine ten major WM tracts for tract-wise differences in WM morphology and microstructural integrity and relationships between the two. Additionally, theories of developmentally predicted trajectories of age-related WM declines, collectively called retrogenesis theory4,9,11, were tested using four ordered models drawn from existing literature. We hypothesized that (1) based on the theory that age-related declines in WM integrity precede changes in WM microstructure, tracts demonstrating more advanced morphometric decline would also have more advanced microstructural decline (2) the pattern of microstructural decline would support the retrogenesis theory of WM decline.Methods
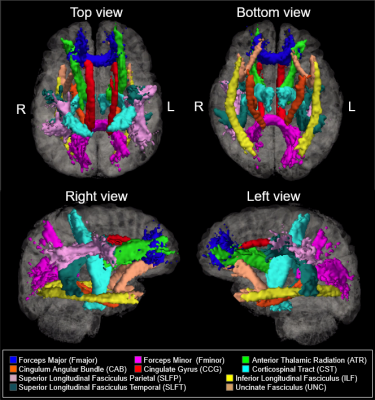
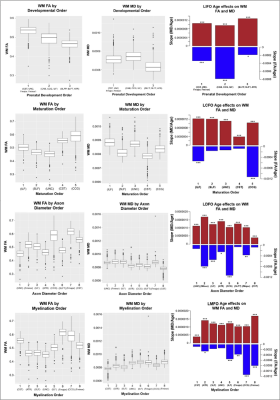
535 healthy adult subjects (300 female, aged 36-100) were drawn from the Human Connectome Project in Aging (HCP-A) dataset (OMB Control# 0925-0667)3. All subjects were in generally good health and without pathological cognitive impairment (i.e. stroke, clinical dementia). We accessed whole-brain T1-weighted structural MRI, as well as diffusion-weighted MRI (dMRI) data collected using four matched Siemens Prisma 3T MRI scanners, with a (1.5mm)3 voxel resolution, MB=4, with 93 directions at b=1500s/mm2. dMRI data were corrected for eddy-current and susceptibility-related distortions via EDDY. FA and MD maps were then derived using Dipy’s DKI tool, which provides kurtosis-corrected DTI metrics (given the high b-value used). Diffusion data were used to identify and reconstruct eighteen major white matter tracts using the Tracts Constrained by Underlying Anatomy (TRACULA) tool in Freesurfer version 7.2.011,13. FSL’s fslmaths function was used to produce subject-specific masks of WM tracts in native space12. The eighteen reconstructed tracts were combined bilaterally where possible to produce ten tracts of interest for analysis. The morphological metrics include tract volume, length, the ratio of volume to length (VLR), whereas microstructural metrics include fractional anisotropy (FA) and mean diffusivity (MD). All analyses in this study were conducted in R (version 4.1.1). Relationships between baseline morphometric/microstructural values, age, and sex were assessed using multivariate regression to first assess relationships in whole-brain comparisons, then on a tract-by-tract basis. Subsequently, four retrogenesis models were tested to address tractwise FA and MD based on order of prenatal development7, maturation14, myelination6, and axon diameter8 respectively.Results
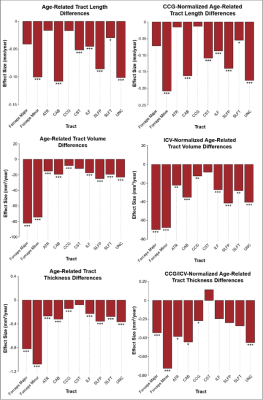
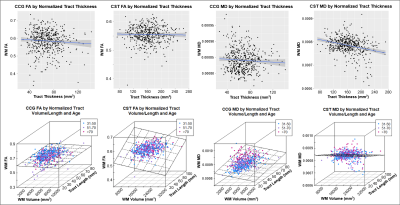
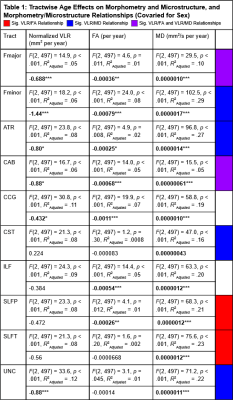
Results indicated wide variation in rates and patterns of morphological and microstructural decline across tracts. While consistent age-related declines in both VLR and microstructural integrity were identified in the majority of tested tracts, relationships between morphometry and microstructure were inconsistently related, with some tracts demonstrating microstructural declines in the absence of morphological differences and others exhibiting age-related morphometry differences with no significant declines in microstructural integrity (Table 1)(Figure 1). Robust sex differences were also identified in these relationships.Tractwise comparisons demonstrated a wide range of relationships between morphometry and microstructure in aging when accounting for sex differences (Figure 2). Moreover, tract length and VLR were related to whole-brain MD in multiple analysis models, while FA associated less consistently with morphometry (Table 1). Additionally, assessment of retrogenesis theory found little support by our aging-related WM variations, although all four ordered models could be used to identify differences in the extent of WM microstructural decline across tracts (Figure 3).Discussion
Our first hypothesis that morphological and microstructural variations with age would be identified consistently in tandem across tracts was not substantiated by our data. While some tracts did demonstrate these patterns of relationships between our tested metrics, others demonstrated microstructural variations without proportional morphological variations. These findings suggest the presence of additional factors driving morphometric and microstructural declines individually with age. One variable of interest for future search of the driver of WM decline will be WM perfusion. Moreover, our findings did not fully support any of the existing retrogenesis predictions of tract-wise aging trajectories. While all models could be used to explain some of the differences between tracts, no clear predictive relationships with developmental order were observed consistently in any of the models. Existing research has demonstrated mixed support for retrogenesis in terms of a “last-in-first-out” order of WM decline4, though some studies have shown anterior-posterior rather than purely tract-driven trends in rate of decline9,11. For example, there appears to be support for an anterior-posterior difference when comparing the forceps major and forceps minor specifically, but to characterize whole tracts as “last in” or “first in” does not seem to provide insight in to the WM degeneration trajectory in normal aging. This suggests that retrogenesis may be better studied within tracts, rather than across tracts.Acknowledgements
This study was supported through grant funding by the Canadian Institutes of Health Research (CIHR)
We would like to thank the Human Connectome Project in Aging for the use of their data in this study, as well as the Laboratory for Computational Neuroimaging for their assistance and continued work on the Freesurfer package.
References
1. Baker, L. M., Laidlaw, D. H., Conturo, T. E., Hogan, J., Zhao, Y., Luo, X., Correia, S., Cabeen, R., Lane, E. M., Heaps, J. M., Bolzenius, J., Salminen, L. E., Akbudak, E., McMichael, A. R., Usher, C., Behrman, A., & Paul, R. H. (2014). White matter changes with age utilizing quantitative diffusion MRI. Neurology, 83(3), 247–252.
2. Bastin, M. E., Muñoz Maniega, S., Ferguson, K. J., Brown, L. J., Wardlaw, J. M., MacLullich, A. M., & Clayden, J. D. (2010). Quantifying the effects of normal ageing on white matter structure using unsupervised tract shape modelling. NeuroImage, 51(1), 1–10. https://doi.org/10.1016/j.neuroimage.2010.02.036
3. Bookheimer, S.Y., Salat, D.H., Terpstra, M., Ances, B.M., Barch, D.M., Buckner, R.L., Burgess, G.C., Curtiss, S.W., Diaz-Santos, M., Elam, J.S., Fischl, B., Greve, D.N., Hagy, H.A., Harms, M.P., Hatch, O.M., Hedden, T., Hodge, C., Japardi, K.C., Kuhn, T.P., Ly, T.K., Smith, S.M., Somerville, L.H., Uğurbil, K., van der Kouwe, A., Van Essen, D., Woods, R.P., Yacoub, E., 2019. The Lifespan Human Connectome Project in Aging: An overview. Neuroimage 185, 335–348.
4. Brickman, A. M., Meier, I. B., Korgaonkar, M. S., Provenzano, F. A., Grieve, S. M., Siedlecki, K. L., Wasserman, B. T., Williams, L. M., & Zimmerman, M. E. (2012). Testing the white matter retrogenesis hypothesis of cognitive aging. Neurobiology of Aging, 33(8), 1699–1715.
5. Choy, S. W., Bagarinao, E., Watanabe, H., Ho, E., Maesawa, S., Mori, D., Hara, K., Kawabata, K., Yoneyama, N., Ohdake, R., Imai, K., Masuda, M., Yokoi, T., Ogura, A., Taoka, T., Koyama, S., Tanabe, H. C., Katsuno, M., Wakabayashi, T., Kuzuya, M., … Sobue, G. (2020). Changes in white matter fiber density and morphology across the adult lifespan: A cross-sectional fixel-based analysis. Human brain mapping, 41(12), 3198–3211. https://doi.org/10.1002/hbm.25008
6. Grotheer, M., Rosenke, M., Wu, H., Kular, H., Querdasi, F. R., Natu, V. S., Yeatman, J. D., & Grill-Spector, K. (2022). White matter myelination during early infancy is linked to spatial gradients and myelin content at birth. Nature Communications, 13(1), 997.
7. Huang, H., Xue, R., Zhang, J., Ren, T., Richards, L. J., Yarowsky, P., Miller, M. I., & Mori, S. (2009). Anatomical characterization of human fetal brain development with diffusion tensor magnetic resonance imaging. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 29(13), 4263–4273.
8. Huang, S. Y., Tian, Q., Fan, Q., Witzel, T., Wichtmann, B., McNab, J. A., Daniel Bireley, J., Machado, N., Klawiter, E. C., Mekkaoui, C., Wald, L. L., & Nummenmaa, A. (2020). High-gradient diffusion MRI reveals distinct estimates of axon diameter index within different white matter tracts in the in vivo human brain. Brain Structure & Function, 225(4), 1277–1291.
9. Kiely, M., Triebswetter, C., Cortina, L. E., Gong, Z., Alsameen, M. H., Spencer, R. G., & Bouhrara, M. (2022). Insights into human cerebral white matter maturation and degeneration across the adult lifespan. NeuroImage, 247, 118727.
10. Maffei, C., Lee, C., Planich, M., Ramprasad, M., Ravi, N., Trainor, D., Urban, Z., Kim, M., Jones, R., Henin, A., Hofmann, S., Pizzagalli, D., Auerbach, R., Gabrieli, J., Whitfield-Gabrieli, S., Greve, D., Haber, N., Yendiki, A. Using diffusion MRI data acquired with ultra-high gradients to improve tractography in routine-quality data. bioRxiv 2021.06.28.450265; doi: https://doi.org/10.1101/2021.06.28.450265
11. Slater, D. A., Melie-Garcia, L., Preisig, M., Kherif, F., Lutti, A., & Draganski, B. (2019). Evolution of white matter tract microstructure across the life span. Human Brain Mapping, 40(7), 2252–2268.
12. Smith, S.M., Jenkinson, M., Woolrich, M.W., Beckmann, C.F., Behrens,
T.E.J., Johansen-Berg, H., Bannister, P.R., De Luca, M., Drobnjak, I., Flitney,
D.E., Niazy, R., Saunders, J., Vickers, J., Zhang, Y., De Stefano, N., Brady,
J.M., and Matthews, P.M. Advances in functional and structural MR image
analysis and implementation as FSL. NeuroImage, 23(S1):208-19, 2004
13. Yendiki, A., Panneck, P., Srinivasan, P., Stevens, A., Zöllei, L., Augustinack, J., Wang, R., Salat, D., Ehrlich, S., Behrens, T., Jbabdi, S., Gollub, R., & Fischl, B. (2011). Automated probabilistic reconstruction of white-matter pathways in health and disease using an atlas of the underlying anatomy. Frontiers in neuroinformatics, 5, 23. https://doi.org/10.3389/fninf.2011.00023
14. Yu, Q., Peng, Y., Kang, H., Peng, Q., Ouyang, M., Slinger, M., Hu, D., Shou, H., Fang, F., & Huang, H. (2020). Differential White Matter Maturation from Birth to 8 Years of Age. Cerebral Cortex , 30(4), 2673–2689.
Figures