1306
Brain-wide fMRI Connectivity and Regional Genetic Modulations underlying Optogenetically-evoked Spindles in Rescuing Memory Decline in Aging1Laboratory of Biomedical Imaging and Signal Processing, The University of Hong Kong, Hong Kong SAR, China, 2Department of Electrical and Electronic Engineering, The University of Hong Kong, Hong Kong SAR, China, 3School of Biomedical Sciences, The University of Hong Kong, Hong Kong SAR, China
Synopsis
Keywords: Neurodegeneration, Aging
Memory consolidation, the ability to transform newly learned information into long-term memory, declines with age. Our previous study revealed targeted neuromodulation of spindle activities can arrest memory consolidation dysfunction in aging brains through strengthening multi-target memory representations. However, whether and how spindle activities influence memory consolidation via acting on inter-regional information integration remained unclear. Here, we demonstrate in aging animals that optogenetically-evoked spindle activities alleviate memory consolidation dysfunction through modulating brain-wide inter-regional connectivity and regional genetic expression. Our work provides an approach combining fMRI analysis and genetic expression profiling to bridge systems- and molecular-level understandings of memory consolidation.Introduction
A half century of learning and memory studies in animal models have demonstrated the underpinning gene-expression-dependent molecular and cellular processes from individual synaptic connection level to meso-scale neural circuitry level 1,2. In the past 25 years, our understanding of systems level memory consolidation over brain-wide networks has also been substantially improved, mainly by studying human and animal brains in vivo with evolving fMRI and electrophysiology methodologies 1,3,4. Guided by such knowledge, tremendous efforts have been devoted to developing activity mapping tools for imaging memory-associated whole-brain genetic actions, via combining activity-dependent genetic labeling with advanced microscopy in extracted brains 5-8. These approaches rely on a selected gene acted immediately during memory activities and are largely limited by the time point of brain extraction and by the structurally-defined neural circuits. Thus, a huge gap remained between such immediate genetic activity mapping and consequential dynamical systems level functional interactions informed by fMRI and electrophysiology studies.Spindle oscillatory activities have been highlighted in mediating system memory consolidation by spatiotemporally orchestrating distributed neuronal activities 3,4,9,10, while the molecular and cellular level underpinnings remain unknown. Previously, through combining optogenetic fMRI, visual fMRI (vfMRI) and behavioral tests, we successfully revealed brain-wide propagation targets of thalamically-evoked spindle activities and their critical sites of systems level action for facilitating memory consolidation in normal rats 11,12 and alleviating memory decline in accelerated aging rats 13. Here, we explore an approach combining brain-wide fMRI functional connectivity analysis and regional gene expression profiling in aging animal model to reveal the potential spindle-associated genetic actions in system memory consolidation.
Methods
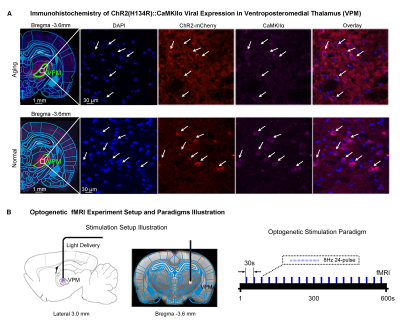
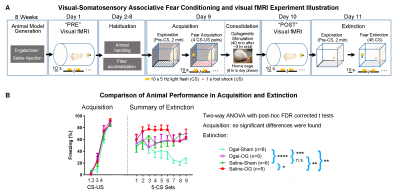
Animal preparation and MRI experimental setup: 3μl AAV5-CaMKIIα::ChR2(H134R)-mCherry was injected to ventral posteromedial (VPM) thalamus of adult SD rats (6 weeks). After 4 weeks, animals were injected with D-galactose daily for 8 weeks to induce accelerated aging14. Optical fiber cannulas were implanted at the injection sites (Figure 1A). fMRI experiments were performed at 7T scanner under 1.0% isoflurane. 8Hz 24-pulses optogenetic stimulation were used to evoke spindle activities 11,12,15,16 (Figure 1B).Visual fMRI, behavior experiments and data analysis: Optogenetic (OG) and Sham animals acquired visual-somatosensory associative memory via receiving 10s 5Hz light flash (CS, conditioned stimulus) co-terminated with foot shock (US, unconditioned stimulus). Animals received 40min of VPM stimulation during memory consolidation. VfMRI experiments were conducted before (day 1, PRE) and after (day 10, POST) animals underwent the behavioral training. Memory performance was assessed by measuring animal freezing levels against 45 repetitions of CS at day 11 (Figure 2A). Seed-based fMRI functional connectivity were analyzed after regressing ROI-averaged vfMRI activations.
Genetic expression profiling: Guided by fMRI results, brain tissues in key regions were extracted on Day 12 and were analyzed using Next-Generation-Sequencing method to identify memory-related genes expressions significantly (p<0.05) regulated with OG compared to Sham.
Results
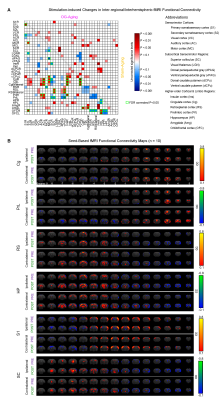
Optogenetically-evoked brain-wide spindle activities rescue the declined memory consolidation in aging animals (Figure 2B): We found that visual-somatosensory associative memory in Sham-Aging animals declined significantly compared to Sham-Normal. Memory performance of OG-Normal animals was significantly enhanced compared to Sham-Normal. Evoking brain-wide spindle activities in OG-Aging animals significantly rescued such impaired memory performance.Modulation of brain-wide inter-regional fMRI functional connectivity underlies alleviated associative memory consolidation in aging animals: We found that OG-Aging animals showed significantly modulated (enhanced or decreased) inter-regional functional connectivity between multiple key limbic (cingulate, prelimbic, retrosplenial, insular, orbitofrontal, amygdala, hippocampus) and sensorimotor (somatosensory, visual, auditory and motor cortices, visual thalamus, superior colliculus, periaqueductal gray, ventral caudate putamen) regions compared to baseline (Figure 3). Significant decreases were found mainly between hippocampus, frontal cortices and superior colliculus. Meanwhile, Sham-Aging group only displayed trends of modulation in functional connectivity between these regions.
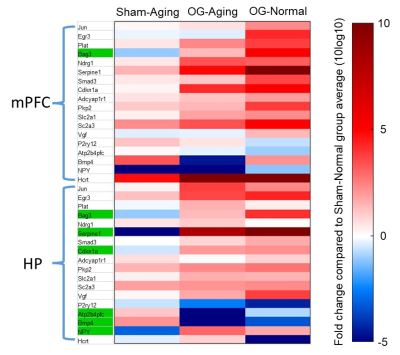
Regulation of multiple memory-related genes in key regions: We found that OG-Aging and OG-Normal groups displayed consistent up- or down-regulation trends across multiple memory-related genes in medial prefrontal cortex and hippocampus compared to Sham-Normal (Figure 4). Compared to Sham-Aging, OG-Aging and Normal animals exhibited a consistently reversed direction of modulation in 6 genes at hippocampus but only in 1 gene at medial prefrontal cortex.
Discussion and Conclusion
Our study demonstrates that optogenetically-evoked spindle activities enhanced memory consolidation in aging animals, specifically through modulation of brain-wide inter-regional functional connectivity. Such functional connectivity modulation shares similarities with our previous demonstration of brain-wide response potentiation13 in terms of the modulated regions. We also found some regions beyond those displaying response potentiation, including hippocampus, periaqueductal gray, ventral caudate putamen and auditory cortex. This indicates that spindle activities modulate large-scale inter-regional functional connectivity over key brain-wide targets overlapping with, but not identical to, memory representation strengthening targets to shape information integration. Sham-Aging animals showed no significant modulation of functional connectivity albeit response potentiation was found in limited sensorimotor and limbic regions13. This could be due to aging-related decrease of spindle propagation13. Further, we selectively screen gene expression changes in two key regions and found multiple genes reversely regulated by aging and OG, mainly in hippocampus. This and future analyses of other key regions may help to understand the modulation of large-scale inter-regional information integration. In conclusion, this study reveals the action of spindle activities on inter-regional network connectivity and demonstrates an approach toward the further examination of genetic consequences in key regions guided by fMRI.Acknowledgements
This work was supported in part by Hong Kong Research Grant Council (HKU17112120, HKU17127121, HKU17127022 and R7003-19F to E.X.W., and HKU17103819, HKU17104020 and HKU17127021 to A.T.L.L.), Lam Woo Foundation, and Guangdong Key Technologies for AD Diagnostic and Treatment of Brain (2018B030336001) to E.X.W..References
1. Kandel, E.R., Dudai, Y. & Mayford, M.R. The molecular and systems biology of memory. Cell 157, 163-186 (2014).
2. Josselyn, S.A. & Tonegawa, S. Memory engrams: Recalling the past and imagining the future. Science 367 (2020).
3. Dudai, Y., Karni, A. & Born, J. The consolidation and transformation of memory. Neuron 88, 20-32 (2015).
4. Klinzing, J.G., Niethard, N. & Born, J. Mechanisms of systems memory consolidation during sleep. Nat Neurosci 22, 1598-1610 (2019).
5. Ye, L., et al. Wiring and Molecular Features of Prefrontal Ensembles Representing Distinct Experiences. Cell 165, 1776-1788 (2016).
6. Vetere, G., et al. Chemogenetic Interrogation of a Brain-wide Fear Memory Network in Mice. Neuron 94, 363-374 e364 (2017).
7. DeNardo, L.A., et al. Temporal evolution of cortical ensembles promoting remote memory retrieval. Nat Neurosci 22, 460-469 (2019).
8. Roy, D.S., et al. Brain-wide mapping reveals that engrams for a single memory are distributed across multiple brain regions. Nat Commun 13, 1799 (2022).
9. Diekelmann, S. & Born, J. The memory function of sleep. Nat Rev Neurosci 11, 114 (2010).
10. Antony, J.W., Schonauer, M., Staresina, B.P. & Cairney, S.A. Sleep Spindles and Memory Reprocessing. Trends Neurosci 42, 1-3 (2019).
11. Wang, X., et al. Functional MRI investigation of Optogenetically-evoked Spindle-like Neural Activity and Memory Consolidation. in Proceedings of International Society of Magnetic Resonance in Medicine, Virtual Conference, p1353 (2020).
12. Wang, X., Leong, A.T.L., Guo, A.S., Dong, C.M. & Wu, E.X. Optogenetically-evoked spindle-like activity from thalamus propagates brain-wide and enhances rsfMRI connectivity. in Proceedings of International Society of Magnetic Resonance in Medicine, Montreal, Canada, p3752 (2019).
13. Wang, X., Leong, A.T., Chong, P.S., Lim, L.-W. & Wu, E.X. Optogenetically-evoked Brain-wide Spindles Alleviate Associative Memory Dysfunction in Aging Animal. in Proceedings of International Society of Magnetic Resonance in Medicine, Virtual Conference, p4668 (Virtual Conference, 2022).
14. Ali, T., Badshah, H., Kim, T.H. & Kim, M.O. Melatonin attenuates D-galactose-induced memory impairment, neuroinflammation and neurodegeneration via RAGE/NF-KB/JNK signaling pathway in aging mouse model. J Pineal Res 58, 71-85 (2015).
15. Fernandez, L.M.J. & Luthi, A. Sleep Spindles: Mechanisms and Functions. Physiol Rev 100, 805-868 (2020).
16. Warby, S.C., et al. Sleep-spindle detection: crowdsourcing and evaluating performance of experts, non-experts and automated methods. Nat Methods 11, 385-392 (2014).
Figures