1305
Medial Temporal Brain Stiffness Predicts Cognition Decline in Aging and Alzheimer's Disease1Department of Radiology, Mayo Clinic, Rochester, MN, United States, 2Department of Physiology and Biomedical Engineering, Mayo Clinic College of Medicine, Rochetser, MN, United States, 3Department of Information Technology, Mayo Clinic, Rochester, MN, United States
Synopsis
Keywords: Neurodegeneration, Aging, Stiffness and Cognition
Aging is associated with neurodegeneration, cognitive function decline, and increased risk of dementia. Objective methods for the longitudinal prediction of cognitive trajectories are needed for design of comprehensive prevention strategies. We tested the hypothesis that measurements of brain mechanical properties will complement existing biomarkers in predicting future cognitive decline. Using linear mixed effect modelling, we evaluated the role of baseline medial temporal stiffness in predicting future cognitive function in participants along the Alzheimer’s disease spectrum.
Target Audience:
Radiologists and neurologists, aging and dementia researchers.Purpose:
To determine whether medial temporal brain stiffness is a predictor of future cognitive decline in aging and Alzheimer's disease.Introduction:
Aging is associated with morphological alterations and functional disruptions in multiple brain regions, leading to cognitive function decline and increased risk of future dementia. Predicting the longitudinal cognitive trajectories for individuals may assist in the design and evaluation of prevention strategies. Brain atrophy, cortical thinning, and microstructural abnormalities including white matter hyperintensities have been shown to predict future incidence of dementia1-3. Medial temporal atrophy has shown to be an important biomarker in predicting the longitudinal cognitive changes in Alzheimer’s disease (AD)4-6. Atrophy is a relatively late event in the aging and AD cascade, so developing advanced imaging methods sensitive to tissue microstructure may enable earlier predictions of cognitive decline7-9. This study assesses the role of medial temporal brain stiffness in predicting the global cognitive decline in aging and Alzheimer's disease groups.Methods:
Study Participants: After obtaining the Institutional Review Board approval and written informed consent from the volunteers and/or their proxies, 63 participants including Amyloid-negative cognitively unimpaired (CU,N=41), Amyloid-positive cognitively unimpaired participants (A+CU,N=13), Amyloid-positive participants with mild cognitive impairment (A+MCI,N=7), Amyloid-positive Alzheimer’s clinical syndrome (A+ACS,N=2) were recruited in this study.Data acquisition and image processing: Participants were scanned on 3T GE scanners (GE, Waukesha, WI) with an 8-channel GE receive-only head coil using previously established methods10. Stiffness maps were computed for each participant using neural network inversion11. Medial temporal (MT) stiffness was calculated using an in-house cortical gray matter region atlas12. Our previous study reported that alterations in MT stiffness are most sensitive to AD pathology and best for discriminating various etiologies of dementia13. Average cortical thickness in a meta-ROI regions for each participant was measured using MPRAGE and FreeSurfer, version v5.3 as described by Schwarz et al.14. A global PET standardized uptake value ratio (PiB-SUVr) was calculated by performing Amyloid PET imaging as described previously15. Using MPRAGE and FLAIR images from each participant, WMH segmentations were performed as previously described16. WMH segmentations were summarized for each participant as the log-transformed volume as a percentage of total intracranial volume. A Cardiovascular and Metabolic Condition (CMC) score was calculated as described previously17. Longitudinal cognitive function evaluation for each participant consisting of a set of neuropsychological battery of tests was performed as described18, 19. Global cognition z-score, computed as the mean of the 4 standardized cognitive domain scores: memory, language, attention/executive, and visuospatial function, was used in the analysis18.
Linear mixed effect modelling and cognition prediction: A set of linear mixed effect models were fit with global cognition z-score as dependent variable. Fixed effect variables used in this study included age (at the time of MRE data acquisition), sex, education and occupation score, cycle number (number of times the cognitive tests were taken to account for the practice effect influence), time (cognition follow-up time or the time from which cognition is first measured), and interaction of age with time. The parsimonious models were reached after removing the non-significant terms and accounting for nested effects iteratively. In the first model, effects of medial temporal stiffness and its interaction with time on the cognition prediction were evaluated. In the second model, additional effects of cortical thickness and its interaction with time on cognition were evaluated. In the third model additional effects of WMH, CMC and PiB-SUVr and their corresponding interactions with the time are added to the second model. The effects of WMH, CMC, PiB-SUvr, cortical thickness and their corresponding time interactions on the cognition were assessed with individual linear mixed models.
Results and Discussion:
Results of three linear mixed models to assess the role of medial temporal stiffness in cognition prediction are summarized in Table 1. In models 1 and 2, medial temporal stiffness and its interaction with time are statistically significant in prediction of cognitive performance whereas in model 3, medial temporal stiffness has a considerable role with P value = 0.051 and its interaction with time has statistically significant role (P <0.05) in the prediction of cognitive performance. Fig.1 shows the global cognition z-scores of all the participants with age. Figs. 2A, 2B shows the global cognition score prediction trajectories (models 1 and 2) with cognition follow-up time, for two participants with a low and high medial temporal stiffness. These results indicate that softer medial temporal stiffness has faster cognitive decline. Overall, stiffness has a significant role in prediction of cognitive score even after controlling for other established biomarkers such as cortical thickness, PiB-SUVr, WMH and CMC. The statistical significance of stiffness as a biomarker is retained even after controlling for cortical thickness, age and its interaction with time and quadratic age. These results suggest that the measurement of stiffness as a biomarker can contribute to predicting cognitive decline independent of the influence of atrophy and aging effects in neurodegeneration.Conclusion:
Brain mechanical alterations predict future cognitive decline and may allow further understanding of tissue microstructural changes associated with cognitive decline in aging and Alzheimer’s disease.Acknowledgements
This work is supported by grants from the NIH, EB027064, EB001981, U01 NS100620 and P50 AG062677.References
1. Mungas D, Reed BR, Jagust WJ, et al. Volumetric MRI predicts rate of cognitive decline related to AD and cerebrovascular disease. Neurology 2002;59:867-873.
2. van der Flier WM, van der Vlies AE, Weverling-Rijnsburger AWE, et al. MRI measures and progression of cognitive decline in nondemented elderly attending a memory clinic. International Journal of Geriatric Psychiatry 2005;20:1060-1066.
3. Dickerson BC, Wolk DA. MRI cortical thickness biomarker predicts AD-like CSF and cognitive decline in normal adults. Neurology 2012;78:84.
4. Jack CR, Jr., Petersen RC, Xu YC, et al. Medial temporal atrophy on MRI in normal aging and very mild Alzheimer's disease. Neurology 1997;49:786-794.
5. Visser PJ, Verhey FRJ, Hofman PAM, Scheltens P, Jolles J. Medial temporal lobe atrophy predicts Alzheimer's disease in patients with minor cognitive impairment. Journal of Neurology, Neurosurgery &amp; Psychiatry 2002;72:491.
6. Chauveau L, Kuhn E, De la Sayette V, Chetelat G, de Flores R. Medial temporal lobe subregional atrophy in ageing and Alzheimer’s disease: A longitudinal study. Alzheimer's & Dementia 2021;17:e051783.
7. Prins ND, Scheltens P. White matter hyperintensities, cognitive impairment and dementia: an update. Nature reviews Neurology 2015;11:157-165.
8. Raghavan S, Przybelski SA, Reid RI, et al. Reduced fractional anisotropy of the genu of the corpus callosum as a cerebrovascular disease marker and predictor of longitudinal cognition in MCI. Neurobiology of aging 2020;96:176-183.
9. Delgorio PL, Hiscox LV, Daugherty AM, et al. Structure–Function Dissociations of Human Hippocampal Subfield Stiffness and Memory Performance. The Journal of Neuroscience 2022;42:7957.
10. Muthupillai R, Lomas DJ, Rossman PJ, Greenleaf JF, Manduca A, Ehman RL. Magnetic resonance elastography by direct visualization of propagating acoustic strain waves. Science (New York, NY) 1995;269:1854-1857.
11. Scott JM, Pavuluri K, Trzasko JD, et al. Impact of material homogeneity assumption on cortical stiffness estimates by MR elastography. Magnetic Resonance in Medicine 2022;88:916-929.
12. Schwarz CG, Gunter JL, Ward CP, et al. [IC-P-122]: THE MAYO CLINIC ADULT LIFE SPAN TEMPLATE: BETTER QUANTIFICATION ACROSS THE LIFE SPAN. Alzheimer's & Dementia 2017;13:P93-P94.
13. Pavuluri K, Scott JM, et al. Differential Effect of Dementia Etiology on Cortical Stiffness as Assessed by MR Elastography. Proc Intl Soc Mag Reson Med; 2022: 3960.
14. Schwarz CG, Gunter JL, Wiste HJ, et al. A large-scale comparison of cortical thickness and volume methods for measuring Alzheimer's disease severity. NeuroImage Clinical 2016;11:802-812.
15. Jack CR, Jr., Wiste HJ, Weigand SD, et al. Defining imaging biomarker cut points for brain aging and Alzheimer's disease. Alzheimer's & dementia : the journal of the Alzheimer's Association 2017;13:205-216.
16. Graff-Radford J, Arenaza-Urquijo EM, Knopman DS, et al. White matter hyperintensities: relationship to amyloid and tau burden. Brain : a journal of neurology 2019;142:2483-2491.
17. Vassilaki M, Aakre JA, Cha RH, et al. Multimorbidity and Risk of Mild Cognitive Impairment. Journal of the American Geriatrics Society 2015;63:1783-1790.
18. Roberts RO, Geda YE, Knopman DS, et al. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology 2008;30:58-69.
19. Vemuri P, Lesnick TG, Przybelski SA et al. Association of Lifetime Intellectual Enrichment With Cognitive Decline in the Older Population. JAMA Neurology 2014, 71:1017-1024
Figures
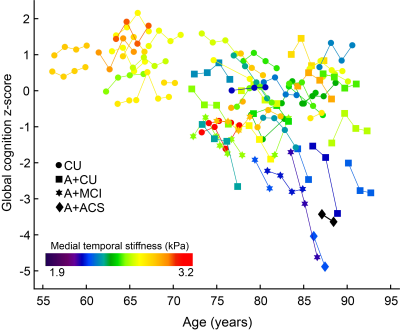
Figure 1: Global cognition z-scores of all the participants with age. Follow-up data points are connected by solid lines. All participants are color coded with their medial temporal stiffness.
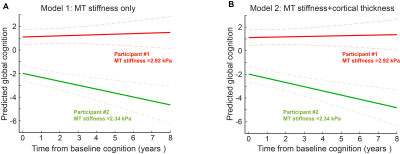
Figure 2: Global cognition predictions with time for 2 participants with a low and high medial temporal stiffness (models 1 and 2). Global cognition z-score prediction trajectories of 2 participants with the cognition follow-up time for medial temporal stiffness only model (A), for medial temporal stiffness and cortical thickness model (B). The softer medial temporal brain has a steeper cognitive decline.
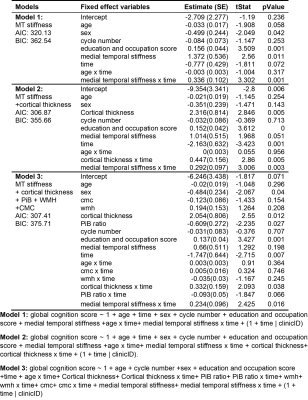
Table 1: Mixed effect models evaluating the utility of medial temporal stiffness in cognition prediction.