1298
A ray of light against age-related Neurodegenerative disease: A 31P Magnetisation Transfer MRS study1Biomolecular Sciences, University of Urbino Carlo Bo, Urbino, Italy, 2Chemistry, University of York, York, United Kingdom, 3Psychology, University of York, York, United Kingdom, 4Sport and Exercise Science, Institute of Science, Manchester Metropolitan University, Manchester, United Kingdom
Synopsis
Keywords: Neurodegeneration, Magnetization transfer, 31P
Through combined theoretical Monte Carlo stimulation and practical 31P Magnetisation Transfer Magnetic Resonance Spectroscopy we quantify the effects of 670 nm photobiomodulation treatment on healthy aging brains. Mitochondrial function declines with age and many pathological processes of neurodegenerative diseases stem from this mitochondrial dysfunction when they fail to produce the necessary energy required. Therefore, an aging population coupled with associated increases in cases of neurological conditions amplifies the need to develop safe, inexpensive treatments to restore mitochondrial function and offer neuronal protection as we grow old. Evidence shows that non-invasive transcranial red/infrared photobiomodulation (PBM) therapy can offer such neuroprotective benefits.Introduction
An ageing global population and subsequent increase in age-related neurological conditions continues to be one of the biggest clinical problems the world is facing. There is a need to develop safe, inexpensive treatments to improve quality of life. Preclinical research has shown that non-invasive transcranial red/infrared photobiomodulation (PBM) offers neuroprotective effects across a wide range of conditions including brain injury, stroke, Alzheimer's and Parkinson's disease, depression, anxiety and age-related cognitive decline. These conditions particularly affect the mitochondria (the 'powerhouses of the cell'), rendering them vulnerable to oxidative stress, reducing energy (adenosine triphosphate, ATP) production and increasing cell death. PBM with red/infrared light has been shown to rescue mitochondrial function and alleviate oxidative stress[1]. Clinical recognition of PBM therapy is currently limited by mechanistic uncertainty underlying its 'positive' neuroprotective effects.Our main aim is to understand and provide evidence for the biochemical/physical mechanism of action of 670nm PBM (red light therapy) of neuro-protection in the human brain. This would deliver two important outcomes: 1) strengthen confidence in PBM as an acceptable healthcare technology to improve human health, and 2) provide an objective, non-invasive metric to assess and optimize its therapeutic efficacy for neurological conditions and age-related cognitive decline.
In this study light transport modelling and 31P Magnetization Transfer Magnetic Resonance Spectroscopy (MT-MRS)[2] were used for quantification of the protective effects of direct photobiomodulation.
The exchange pathways and the exchange species with their associated 31P signals are illustrated in figure 1. Magnetic labelling of the terminal phosphate (γ) of ATP induces a 31P MRS signal decrease in both Pi and PCr . The signal reduction, or either Pi or PCr, as a function of saturation time, τ, follows an exponential decay[3]:
$$S(τ)=S(0)((k_f.T_{app} ) e^{-(τ/T_{app} )} +T_{app}/T_1 ) $$ …[1]
Where, is the forward exchange rate towards ATP, T1 is the intrinsic relaxation time of PCr or Pi, and the ‘apparent’ time constant of decay, Tapp, is described as:
$$ 1/T_{app} = 1/T_1 + k_f $$ ...[2]
By varying saturation time and measuring signal levels, one can estimate the forward exchange rate of ATP for both pathways using equations 1 & 2.
Methods
Ten healthy participants aged 60+ were recruited (mean age = 68 years; age range = 60-85 years; 6 females). A 5-day longitudinal study was designed (figure 2a) with 31P MT-MRS measurements developed to quantify cell metabolites (e.g., Pi, PCr & ATP) before (day 1) and after (day 5) PBM treatment (18W, Red Mini 670, Red Light Man Ltd., Manchester, UK) applied to the visual cortex (days 1-4).A 31P MT-transfer FID based pulse sequence was implemented (figure 2a) with a varying number of 114ms saturation radiofrequency (RF) pulses using a 3T MAGNETOM Prisma system (Siemens Healthcare GmbH, Erlangen, D). A dual channel 1H/31P transmit-receive flexible surface coil (O-XL-HL-030-01150 V03; Rapid Biomedical GmbH, Rimpar, D) was used for RF transmission/detection.
Data was supplemented by image informed computer Monte Carlo simulation of light transport[4] (350-1000 nm) through a simplified five-layer tissue model of the human head providing a quantitative estimate of the amount applied PBM light absorbed within the grey matter. The model consisted of optical layers representing skin, skull, cerebrospinal fluid (CSF), grey matter (GM) and white matter (WM).
Results
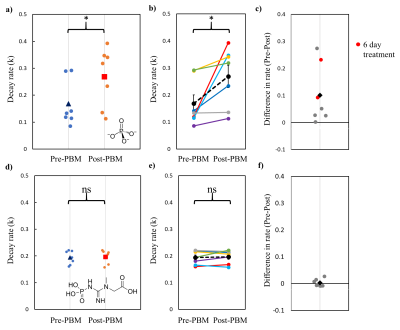
Fitting to the mean decay curve across n=7 participants pre- and post-PBM application (figure 3), we found a kf rate for ATP synthase of 0.158 s-1 and 0.252 s-1 respectively. Fitting each individual decay curve and then averaging extracted kf rates, we found 0.198 ± 0.035 s-1 and 0.298 ± 0.045 s-1 pre- and post-PBM respectively. We found an increase in kf rate, for ATP production post-PBM compared to pre-PBM application. The decay rate for Pi (intracellular) was significantly faster post-PBM compared to pre-PBM (Wilcoxon Signed Ranks test, two-tailed, Z = -2.366, p = 0.016).The relationship between age and Pi k-rate change was not significant (Kendall’s tau b = -0.293, p = 0.362), indicating that age was not a significant confound within the range tested (60 – 85 years). In contrast to Pi, there was no significant change in decay rates of PCr (figure 4) before and after PBM treatment (Wilcoxon Signed Ranks test, two-tailed, Z = -0.338, p = 0.813).
There is a small increase in pH post PBM, 7.54 ± 0.01 compared to 7.50 ± 0.01 pre PBM, this is within standard error and therefore likely not a driver or reflective of the measured increase in ATP synthase flux.
Light modelling through the use of Monte Carlo simulations confirmed the absorption peaks at 670 nm and 820 nm (figure 5). Simulations indicate that any given wavelength (above 450 mm) less than 1% of the incident light reaches the grey matter. This was confirmed in image informed 3D models. We demonstrate a dramatic 2/3 drop in the amount of 670nm photons being absorbed by cytochrome-c in GM under skin with high melanin fraction. The drop is less pronounced (1/2 photon count) at the optimum 820 nm peak.
Discussion and Conclusions
We validated the positive metabolic benefits of PBM in healthy individuals (60-85 years). Next steps include applying this in neurodegenerative disease studies to validate that PBM is indeed a useful neuroprotective technique in aging and disease.Acknowledgements
No acknowledgement found.References
[1] Salehpour, F., J. Mahmoudi, F. Kamari, S. Sadigh-Eteghad, S. H. Rasta, and M. R. Hamblin. 2018. 'Brain Photobiomodulation Therapy: a Narrative Review', Molecular Neurobiology, 55: 6601-36.
[2] Chen, C., M. C. Stephenson, A. Peters, P. G. Morris, S. T. Francis, and P. A. Gowland. 2018. 'P-31 magnetization transfer magnetic resonance spectroscopy: Assessing the activation induced change in cerebral ATP metabolic rates at 3 T', Magnetic Resonance in Medicine, 79: 22-30.
[3] Forsen, S., and R. A. Hoffman. 1963. 'STUDY OF MODERATELY RAPID CHEMICAL EXCHANGE REACTIONS BY MEANS OF NUCLEAR MAGNETIC DOUBLE RESONANCE', Journal of Chemical Physics, 39: 2892-&.
[4] Wang, L. H., S. L. Jacques, and L. Q. Zheng. 1995. 'MCML - MONTE-CARLO MODELING OF LIGHT TRANSPORT IN MULTILAYERED TISSUES', Computer Methods and Programs in Biomedicine, 47: 131-46.
Figures

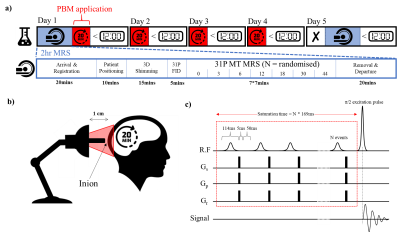
Figure 2: Experimental design for tracking the neuroprotective effects of PBM treatment. a) Experimental Design of 31P MT MRS and PBM therapy b) PBM therapy utilised a 670 nm LED bulb positioned approximately 1 cm from the inion; c) 31P MRS utilised a magnetisation transfer FID based pulse sequence with a varying number of 114 ms saturation RF pulses (0, 3, 6, 12, 18, 30 and 44) followed by a 5 ms spoiler gradient and 50 ms delay. Experiment order was randomised for each scanning session.
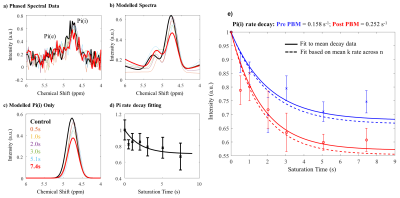
Figure 3: Data analysis pipeline for MT-31P MRS data. a) Raw FID data was filtered and phased before FT into spectral space for baseline correction.; b) Resultant spectra were fitted to appropriate Lorentzian functions for Pi(i) and Pi(e) with chemical shift, amplitude and FWHM free variables; c) Signal contributions from only Pi(i) were isolated; d) Subsequent data were plotted against saturation time and the resulting decay curve fitted to estimate ATP flux rate; e) ATP flux rate estimates pre (blue) and post (red) PBM treatment based on signal decay as a function of RF saturation time.