1289
Highly Accelerated and High-resolution T2 Mapping in the Kidney Based on Echo Merging Plus k-t Undersampling with Reduced Refocusing Flip Angles1The Institute of Science and Technology for Brain-inspired Intelligence, Fudan University, Shanghai, China, 2Department of Radiology, University of Cambridge, Cambridge, United Kingdom, 3Department of Radiology, CUH NHS Foundation Trust, Cambridge, United Kingdom, 4Department of Histopathology, CUH NHS Foundation Trust, Cambridge, United Kingdom, 5Department of Oncology, CUH NHS Foundation Trust, Cambridge, United Kingdom, 6Department of Surgery, CUH NHS Foundation Trust, Cambridge, United Kingdom, 7Sir Peter Mansfield Imaging Centre, University of Nottingham, Nottingham, United Kingdom
Synopsis
Keywords: Kidney, Quantitative Imaging
We developed a highly accelerated multi-echo spin-echo (MESE) method based on echo merging and k-t undersampling with reduced flip angles (TEMPURA), which can be used to either reduce the acquisition time or increase spatial resolution for multi-slice kidney T2 mapping. Compared with a standardized respiratory-gated MESE sequence, fast TEMPURA reduced the acquisition time from 3–5 minutes to one breath-hold (18 s) without degrading measurement accuracy or image quality. It also outperformed using k-t undersampling alone. High-resolution TEMPURA reduced the pixel size from 3×3 mm2 to 1×1 mm2 and greatly improved the visualization of detailed structures.Introduction
T2 mapping is expected to improve the accuracy and reproducibility of detecting and assessing the severity of pathological changes. However, the feasibility of routine T2 mapping has been restricted by the long acquisition time. The acquisition in abdominal regions can be further extended by respiratory triggering, which leads to patient discomfort and motion artefacts. In this study, we developed a highly accelerated multi-echo spin-echo(MESE) method, termed T2 mapping using Echo Merging Plus k-t Undersampling with Reduced refocusing flip Angles(TEMPURA). A fast breath-hold sequence and a high-resolution sequence were both implemented based on TEMPURA. Their performance was compared with a conventional standardized MESE sequence developed for the UK Renal Imaging Network (UKRIN)(1,2) and a fast MESE sequence accelerated by purely k-t undersampling.Methods
The acquisition scheme of TEMPURA is shown in Figure 1. Three adjacent echoes are combined into one k-space, either by combining three independent echoes (echo-combination) or sharing one echo between two k-spaces (echo-sharing). The combined k-space can be then reconstructed based on compressed sensing (CS) theory. Reduced flip angles (175°-145°-110°…-110°) are used for the refocusing pulses to reduce the specific absorption rate, and thus more echoes can be acquired by using minimum echo spacing.Two versions of sequences were developed based on TEMPURA: one highly accelerated breath-hold sequence with reduced TR and one high-resolution sequence using a larger matrix size. Table 1 shows the key parameters of different sequences. A standardized MESE sequence with constant 180° flip angles and SENSE acceleration (x3)(1,2) and a breath-hold sequence accelerated by purely k-t CS undersampling (x9) with reduced flip angles were used for comparison. k-t FOCUSS (3) was used for CS reconstruction. The StimFit toolbox(4,5) based on the extended phase graph algorithm was used as a fitting model with stimulated echo compensation.
All the previously described sequences, together with a NIST reference sequence, were undertaken on the ISMRM/NIST system phantom(6). Acquisitions with acceleration factors from ×3 to ×9 and matrix sizes from 128 to 512 were evaluated respectively. Each sequence was repeated three times.
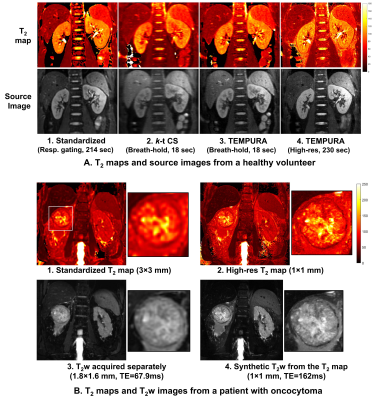
The kidneys of 16 healthy subjects (9 men; 7 women; range 24–47 years) and one patient with renal oncocytoma (male, 73 years) were imaged using a 3 T system (Discovery MR750; GE Healthcare, Waukesha, WI) and a 32-channel cardiac array coil. Breath-hold TEMPURA with echo-sharing (18 s), k-t CS (18 s), and the respiratory-triggered high-resolution TEMPURA (384×384) and standardized sequences were scanned on each subject. A separate T2W 3D FSE sequence (respiratory-triggered, FOV 400×360 mm, matrix 256×224, TE/TR 67.9/8574 ms, ETL 120). ROIs of the cortex, medulla and whole kidney were manually placed on the standardized T2 maps and then applied to the other maps with minor adjustment to correct motions.
Results
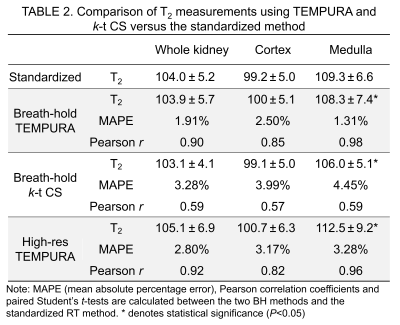
Figure 2 shows the quantitative evaluation of T2 measurements from the NIST phantom using different methods. Among all the three acceleration methods, TEMPURA with echo-sharing has the best accuracy (Fig 2A-2 and C). Compared with the standardized method, the acquisition time can be reduced from 169 to 18 s, with the mean absolute percentage error (MAPE) maintained at a low level (TEMPURA: 8.1% vs. Standardized: 7.35%). TEMPURA with larger matrix sizes greatly improved the visualization of detailed structure (Fig 2A-3, the resolution inset) without increasing the acquisition time. The MAPE of high-resolution TEMPURA is also reduced compared with the standardized method (Fig 2D).Figure 3 shows representative results from a healthy volunteer and a patient diagnosed with oncocytoma. Fast breath-hold TEMPURA achieved similar image quality in comparison with the standardized MESE sequence, whereas k-t CS led to image blurring (Fig 3A). By increasing the resolution by a factor three (in both in-plane directions), high-resolution TEMPURA substantially improved the distinguishability of cortex and medulla (Fig 3A-4) and the visualization of structures within the tumor (Fig 3B-2). A synthetic T2 weighted image can be generated from computed high-resolution T2 and M0 maps, which shows better image quality compared with the T2 weighted images acquired by a separate 3D FSE sequence.
In vivo T2 measurements from the volunteers are shown in Table 2. Using the standardized method as reference, breath-hold and high-resolution TEMPURA both achieved good agreement (MAPE=1.31–2.50% and 2.80–3.28% respectively) and high correlation coefficient (r=0.85–0.98 and 0.82–0.96 respectively), whereas k-t CS showed a much lower correlation (0.57–0.59) and higher MAPE (3.28–4.45%).
Discussion and conclusion
We have presented a highly accelerated and high-resolution MESE method for T2 mapping, TEMPURA, based on k-t undersampling and echo merging. Reduced refocusing flip angles are adopted to increase the echo number, with stimulated echoes corrected in the fitting process.Breath-hold and respiratory-triggered high-resolution renal T2 mapping sequences were developed based on TEMPURA. The breath-hold sequence enables a quick and accurate T2 measurement, which can be potentially used as a quick examination method in the diagnosis of renal diseases. The high-resolution sequence provides a clear depiction of anatomical structures within the kidney, which enables measurements from the cortex and medulla to be distinguished. This high-resolution T2 mapping approach may be used to investigate the morphology and T2 values in disease in the future and could be applied across other body regions.
Acknowledgements
This work was supported by Cancer Research UK, CRUK Cambridge Centre, NIHR Cambridge Biomedical Research Centre, Cambridge Experimental Cancer Medicine Centre, Addenbrooke’s Charitable Trust, and the UKRIN-MAPS Medical Research Council Grant MR/R02264X/1.References
1. Li H, Buchanan CE, Morris DM, et al. Improved Harmonization of Renal T2 Mapping Between Vendors using Stimulated Echo Compensation. In: Proceedings of Joint Annual Meeting ISMRM-ESMRMB & ISMRT 31st Annual Meeting. ; 2022. p. 4409.
2. Buchanan CE, Li H, Morris DM, et al. A Travelling Kidney study using a harmonised multiparametric renal MRI protocol. In: Proceedings of the 27th Annual Meeting of ISMRM, Montreal, Canada. ; 2022. p. 0482.
3. Jung H, Sung K, Nayak KS, Kim EY, Ye JC. K-t FOCUSS: A general compressed sensing framework for high resolution dynamic MRI. Magn. Reson. Med. 2009;61:103–116
4. Lebel RM, Wilman AH. Transverse relaxometry with stimulated echo compensation. Magn. Reson. Med. 2010;64:1005–1014
5. Lebel RM. StimFit: A toolbox for robust T2 mapping with stimulated echo compensation. In: In: Proceedings from the 20th Annual Meeting of ISMRM, Melbourne, Australia. Vol. 37. ; 2012. p. 2558.
6. Stupic KF, Ainslie M, Boss MA, et al. A standard system phantom for magnetic resonance imaging. Magn. Reson. Med. 2021;86:1194–1211
Figures
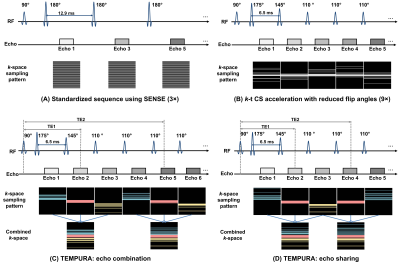
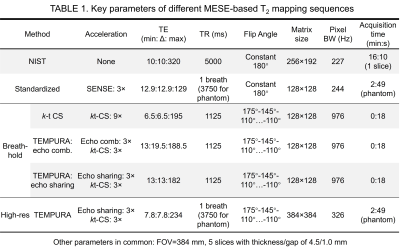
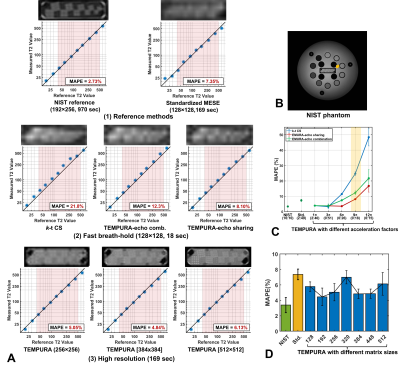
Figure 2. Quantitative evaluation of T2 measurements from the NIST phantom. All the measurements were averaged across three repeated acquisitions. A. Measurements from T2 spheres in comparison with reference values. Red rectangular boxes indicate physiologically meaningful ranges (45–500 ms). Upper images show the resolution inset cropped from corresponding source images. B. NIST phantom with T2 spheres. C. MAPE of T2 measurements using different acceleration methods with different acquisition times. D. MAPE of T2 measurements using TEMPURA with different matrix sizes.