1279
Implantable Coils Enable High-Resolution Functional MRI in Awake Mice
David Hike1, Xiaochen Liu1, Zeping Xie1,2, Bei Zhang1, Wenchao Yang1, Alyssa Murstein1,3, Andy Liu1,3, Daniel Glen4, Richard Reynolds4, and Xin Yu1
1Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Boston, MA, United States, 2School of Traditional Medicine, Southern China University, Guangzhou, China, 3Neuroscience, Boston University, Boston, MA, United States, 4NIMH, National Institutes of Health, Bethesda, MD, United States
1Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Boston, MA, United States, 2School of Traditional Medicine, Southern China University, Guangzhou, China, 3Neuroscience, Boston University, Boston, MA, United States, 4NIMH, National Institutes of Health, Bethesda, MD, United States
Synopsis
Keywords: fMRI (task based), Preclinical, Implantable Coil
This study utilizes implantable RF coils affixed to mouse heads which are used as head fixation points to minimize motion and remove B1-related artifacts due to motion-induced loading change during scanning. This method increases SNR significantly and enables high-resolution EPI-based functional imaging in awake mice at 14T, highlighting both cortical and subcortical activation following visual and whisker stimulation. Furthermore, this high-resolution awake mouse fMRI setup enables high sensitivity to map brain activation from subcortical nuclei and extends the detection of associated brain regions related to the stimulation.Purpose
Functional magnetic resonance imaging (fMRI) can be challenging in preclinical settings when detangling mechanistic questions. Awake mouse fMRI has presented a better opportunity to provide complementary multi-scale brain dynamic information for function-behavioral studies1. Still, motion from animals leads to imaging artifacts, and the spatial resolution is limited due to the voxel volume-determined signal-to-noise ratio (SNR)2,3. To solve these issues, we have implemented a novel implantable radiofrequency (RF) coil as headpost contact for head-fixed awake mouse fMRI, which can effectively eliminate any motion-induced B1-related artifacts. In particular, the mini-RF coils were designed to be lightweight enough for animals to quickly recover after surgery. Here, we report high-resolution whole brain fMRI (with the potential to reach 100um isotropic) following both visual and whisker stimulation, showing robust cortical and thalamic responses using a 14T scanner. For the whisker stimulation, robust cerebellum and hippocampal activation can be detected in awake mouse brains, highlighting the unique global function-behavioral mapping capability of this novel setup. This setup can also be used to record real-time pupillometry and whiskering movementto use as regressors providing a behavior-driven mapping tool to study diseased animal models, e.g. AD.Materials and Methods
Animal Model: C57BL/6J mice (25-30g) underwent a surface coil implantation. Two coil types were implanted and compared with a commercial coil used as a control at 9.4T: 400MHz Single loop coil (9.4T), 600MHz Single loop coil (14T), 400MHz “Figure 8” coil (9.4T), 600MHz “Figure 8” coil (14T) (Figure 1). To implant the coils, mice were induced with 5% and maintained at 2% isoflurane. An incision was made to expose and dry the skull clearing the area of fatty layers and tissue. The coil was positioned over the target area 1mm above the skull and attached using cyanoacrylate glue and 2-part dental cement. Once the glue had hardened, the animal was allowed to recover and placed back in its home cage.MR Techniques: Using both 9.4T and 14T horizontal bore magnets at the Athinoula A. Martinos center and implantable 1H single loop/figure 8 surface coils, high-resolution images were acquired in awake mice. Stimulated fMRI data was acquired with a multi-slice 2D EPI scan with two segments. Each mouse was imaged with: in-plane resolution=100x100µm, matrix size=144x96, slice thickness=200µm, TE/TR=6.2ms/1 s, and 205 repetitions for 6min50s acquisition time (2 s/repetition). Visual stimulation block design consists of a “1 on, 19 off” stimulation paradigm repeated ten times with five baseline scans. Optical stimulation wavelengths 530nm at 5Hz and 490nm at 5.1Hz flashed for 8 seconds over the right eye. Whisker stimulation used an identical block design but sent 10ms air puffs at 8Hz for 8s over the left whisker area. Anatomical images for fMRI and SNR comparisons were acquired at 14T and 9.4T using multi-slice T1-weighted 2D gradient echo Fast Low Angle Shot (FLASH) and identical implantable coils. Each mouse was scanned with: in-plane resolution=100x100µm, matrix size=144x96, slice thickness=400µm, TE/TR=3ms/475ms, 30o flip angle, and 4 averages for 4.5min acquisition time.
Data Analysis: SNR was computed by dividing standard deviation over mean signal. fMRI was processed using Analysis of Functional Neuroimages (AFNI)4. Bruker 2dseq images were converted to AFNI format using ‘to3d’ before masking and aligning the dataset to a template. Data was then despiked, motion corrected, and warped to match the template space. Blurring and scaling was performed before running a linear regression. A clustering threshold was set at 50 voxels and the Pearson correlation values were limited to p<0.05.
Results and Discussion
SNR comparisons of the implantable single-loop coils to a commercially available 4-array coil at 9.4 T show significantly higher SNR with a factor of 2 in the 2D FLASH images of anesthetized mice (Fig 1). Furthermore, when we used a 14T scanner, the SNR is further increased to another 1.5 times. Besides the single-loop coil, we also demonstrated a significantly higher SNR of the figure 8 coil based on different B1 modules to focus on the cortex with up to 6 times higher SNR than the 4-array coil at 9.4T. This setup enables the high spatial resolution fMRI of awake mice with little B1 interference of motion-induced loading changes, and a plug-and-play working environment to significantly reduce the preparation time to ease the training process and reduce the stress of animals(Fig 2). Figure 3 shows the whole brain fMRI with visual stimulation, presenting a strong positive BOLD response in the superior colliculus (SC), lateral geniculate nucleus (LGN), and visual cortex. And Figure 4 shows the whole brain fMRI with whisker stimulation, showing positive BOLD responses in the contralateral barrel cortex and the ventro posteromedial nucleus (VPM), as well as activation in the hippocampus and cerebellum areas. The high spatial resolution fMRI of awake mice with implanted RF coils offers sufficient sensitivity to enable the global mapping of the brain function beyond the typical peripheral ascending pathways. This is best exemplified by the whisker stimulation paradigm. It should be noted that we only highlighted the positive BOLD signals, but not showed the negative BOLD signal from the ipsilateral hemisphere.Conclusion
We introduced a robust awake mouse fMRI environment with implanted RF coils. High spatial resolution fMRI datasets show higher sensitivity to map subcortical brain activation in head-fixed awake-behaving mice.Acknowledgements
This work was supported by the US National Institutes of Health (RF1NS113278, RF1NS124778, R01NS122904, R01NS120594, R21NS121642, P41EB015896, S10RR023043, S10RR023401) the National Science Foundation(2123971), and the Athinoula A. Martinos Center’s instrumentation grant (S10 MH124733–01).References
1. Fonseca, M.S., Bergomi, M.G., Mainen, Z.F. and Shemesh, N., 2022. Functional MRI of large scale activity in behaving mice. bioRxiv, pp.2020-04.
2. Liu, Y., Perez, P.D., Ma, Z., Ma, Z., Dopfel, D., Cramer, S., Tu, W. and Zhang, N., 2020. An open database of resting-state fMRI in awake rats. NeuroImage, 220, p.117094.
3. Uğurbil, K., 2021. Ultrahigh field and ultrahigh resolution fMRI. Current Opinion in Biomedical Engineering, 18, p.100288.
4. RW Cox. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, 29:162-173, 1996.
Figures
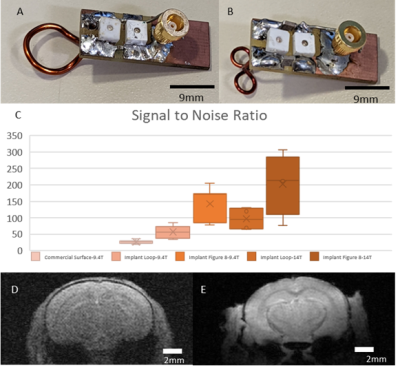
Figure 1: A&B) Representative samples of
the implanted coil designs used for the awake animal imaging. Coils are
miniturized and lightweight to reduce recovery time of animals post implant. C)
shows the much-improved SNR measured at the cortex (~2x for single loop, and
~3x for figure 8 at 9.4T). At 14T, the SNR improved ~1.5x over the comparable
9.4T scan as would be expected with identical scan parameters. D & E shows
the clear visual difference between the standard commercial and implant (A&B)
coils.

Figure 2: Awake mouse imaging setup with both optical and
pneumatic stimulation capabilities and camera mount for recording behavior
during imaging
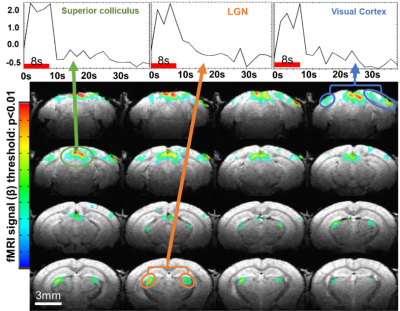
Figure 3: Functional activation seen in awake fMRI following
visual stimulation. Active regions show positive BOLD response following the 5Hz
flashing signal for 8s primarily in visual associative areas
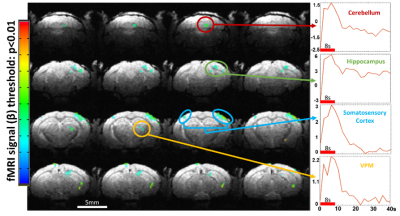
Figure 4: Activation map from whisker stimulation in awake mouse
fMRI. Multiple regions show positive response. Regions of note are the contralateral barrel cortex and the ventro posteromedial
nucleus of the thalamus (VPM). Interestingly, activation in the hippocampus and
cerebellum areas are seen showing association with peripheral areas
DOI: https://doi.org/10.58530/2023/1279