1278
Rapid and high-resolution bSSFP fMRI using 3D stack of spirals at 9.4 T1Magnetic Resonance Center, Max Planck Institute for Biological Cybernetics, Tübingen, Germany, 2German Center for Neurodegenerative Diseases (DZNE), Bonn, Germany, 3Department of Biomedical Magnetic Resonance, Eberhard Karls University, Tübingen, Germany
Synopsis
Keywords: Data Acquisition, fMRI (task based), bSSFP, Spiral, non-cartesian, sub-millimeter, whole-brain
We explored the spatio-temporal resolution and coverage of bSSFP BOLD contrast using a highly segmented 3D spiral readout. The functional activation maps acquired with the novel functional contrast for full visual field checkerboard stimulus is presented at submillimeter resolutions (0.6 mm3 & 0.8 mm3) and at 1.2 mm3 for whole brain coverage. We also demonstrated rapid slice-selective water excitation using binomial pulses.
Introduction
The vessel size specificity of bSSFP-BOLD contrast similar to spin-echo contrast is attractive to high-resolution functional studies at high field strengths, where the former offers flexibility and low RF energy deposition1,2. Several attempts were made, including the previous work3, to improve the speed of bSSFP acquisition using a high duty cycle readout4–6. In addition to optimizing the spiral readout, we investigated rapid water excitation using binomial pulses to improve the image quality of longer readouts at 9.4 T.Methods
Water excitation:For rapid water excitation, two half flip angle slab-selective pulses (binomial-11)7 with bipolar gradients are used as illustrated in Figure 1. The shorter first order water excitation (WE1), where the fat precesses π radian between the pulses, is used for the whole head protocol and the longer second order water excitation (WE2), where the fat precesses 3π radian, is used for the sub-millimeter protocols with thin slabs.
Acquisition:
The improved sequence acquires Cartesian reference data for coil sensitivities in a pre-scan with matched slice profile followed by image acquisition with water-selective excitation as described above. For the functional experiments, three healthy subject were scanned with a human 9.4T scanner (Siemens Healthineers, Erlangen, Germany) equipped with a SC72 whole-body gradient and a home-built 16Tx/31Rx RF Coil8. For all protocols, spatial B0 field map is acquired after every functional scan.
Whole head protocol: TR/TE=5/1 ms, FOV=220x220x172.8 mm3 at 1.2 mm isotropic, RF= 13°/2x280 μs/TBWP=4/ WE1, SENSE=4x2, Segmentation (InterleavesxPartitions)=8x72 of 3.14 ms readouts, volume TR=2.88 s, 140 volumes.
0.8 mm3 isotropic protocol: TR/TE=10/1.6 ms, FOV=210x210x22.4 mm3, RF= 20°/2x400 μs/TBWP=6.2/WE2,SENSE=4, Segmentation (InterleavesxPartitions)=7x28 of 6.54 ms readouts, volume TR=1.96 s, 240 volumes.
0.6 mm3 isotropic protocol: TR/TE=10/1.8 ms, FOV=200x200x10.8 mm3, RF= 20°/2x380 μs/TBWP=5/WE2, SENSE=4, Segmentation (InterleavesxPartitions)=12x18 of 6.03 ms readouts, volume TR=2.16 s, 160 volumes.
Paradigm, Reconstruction and fMRI Analysis:
A full visual field flickering checkerboard pattern was presented during the acquisition period of 10 volumes with equal periods of rest in between for all three experiments. The acquired data was reconstructed with gradient imperfection and static spatial B0 correction using cg-MTI-SENSE algorithm3. The reconstructed volumes are motion corrected in AFNI9, mildly smoothed (filter FWHM= resolution) and GLM fitted in FSL10 and co-registered with additionally acquired anatomical images in SPM11.
Results
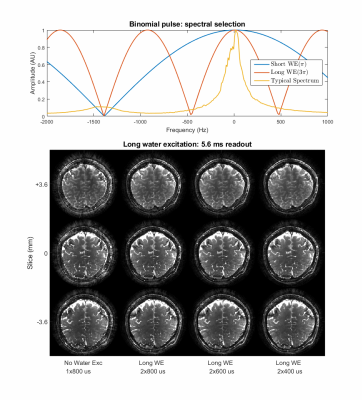
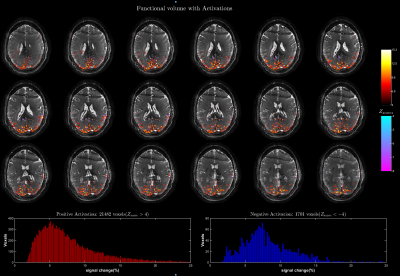
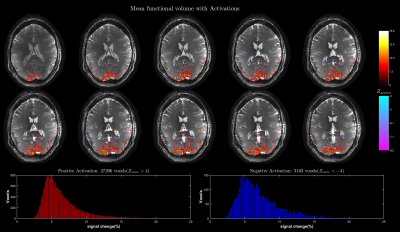
Figure 2 shows the performance of water excitation (WE2) for different RF pulse duration and readout lengths. The results of the functional experiments using the aforementioned three protocols are shown in Figure 3-5 respectively.Discussion
At 9.4T, fat takes about 350 μs to accumulate a phase shift of π, which is not sufficient time to excite thin slices. Therefore, we used a WE2 excitation with an inter-pulse duration of 1050 μs for the high resolution protocols to accommodate gradient switching and high flip angle RF pulse. The poor spectral selection of WE2 is partially compensated by higher spatial B0 homogeneity in thin slabs. Nevertheless, the partial fat suppression significantly improves the image quality as depicted in Figure 2. The 16 parallel transmit setup in CP-mode shows significant flip angle variation resulting in only about 60-70% of nominal flip angle in the occipital region. All protocols are running at slightly sub-optimal flip angle for SNR due to SAR restrictions when the optimum is around 15o for gray matter at 9.4 T.All protocols consistently achieved about 60-65% readout duty cycle (i.e total ADC time divided by total acquisition time), which is close to a gradient-echo EPI acquisition (around 70%) and substantially faster than single-echo Cartesian bSSFP (around 30%). Additional 5% of achievable ADC duty cycle with the current gradient hardware is locked behind the peripheral nerve stimulation limit. In addition to the in-plane acceleration factor of 4, the large coverage of the whole brain protocol allows for an additional parallel imaging factor of two in slice direction.
Highly significant activations localized along gray matter gyri with visually no distortion are observed in all experiments. The expected increase in signal change with increasing TR is evident when comparing the signal change histograms of the whole brain (TR= 5 ms) and the high resolution(TR= 10 ms) results12. Although a TR of 10 ms is adequate for refocusing the extravascular static dephasing component13 with a dispersion of 50 Hz at 9.4T1, a high signal change is observed in voxels near large vessels in the high resolution activation maps. This signal bias could be a result of increased T2* weighting due to long readouts and/or an intravascular signal component, and requires further investigation. The high spatial B0 homogeneity requirement for larger TR (TR= 10 ms requires ΔB0<100 Hz for banding-free results) limits the possible study regions to occipital and superior regions away from large air cavities.
Conclusion
We demonstrated the speed and flexibility of passband bSSFP contrast when combined with accelerated 3D stack-of-spirals readouts for functional imaging at ultrahigh field.Acknowledgements
The financial support of Max-Planck society and Federal Ministry of Education and Research (PSKYBENIBS BMBF-CRCNS MRZ) is gratefully acknowledged.References
1. Scheffler K, Ehses P. High-resolution mapping of neuronal activation with balanced SSFP at 9.4 tesla. Magn Reson Med. 2016;76(1):163-171. doi:10.1002/mrm.25890
2. Scheffler K, Heule R, Báez-Yánez MG, Kardatzki B, Lohmann G. The BOLD sensitivity of rapid steady-state sequences. Magn Reson Med. 2019;81(4):2526-2535. doi:10.1002/mrm.27585
3. Iyyappan Valsala P, Ehses P, Veldmann M, Scheffler K. Accelerated 3D stack-of-spiral bSSFP for functional imaging at 9.4T: Pilot study. #1102, Proc. ISMRM 2022. https://cds.ismrm.org/protected/22MPresentations/abstracts/1102.html
4. Ehses P, Scheffler K. Multiline balanced SSFP for rapid functional imaging at ultrahigh field. Magn Reson Med. 2018;79(2):994-1000. doi:10.1002/mrm.26761
5. Miller KL, Smith SM, Jezzard P, Wiggins GC, Wiggins CJ. Signal and noise characteristics of SSFP FMRI: A comparison with GRE at multiple field strengths. NeuroImage. 2007;37(4):1227-1236. doi:10.1016/j.neuroimage.2007.06.024
6. Veldmann M, Ehses P, Stöcker T. Spiral in/out trajectory with GIRF prediction for passband bSSFP fMRI at 7T,ESMRMB 2020 Meeting.
7. Hore PJ. Solvent suppression in fourier transform nuclear magnetic resonance. J Magn Reson 1969. 1983;55(2):283-300. doi:10.1016/0022-2364(83)90240-8
8. Shajan G, Kozlov M, Hoffmann J, Turner R, Scheffler K, Pohmann R. A 16-channel dual-row transmit array in combination with a 31-element receive array for human brain imaging at 9.4 T. Magn Reson Med. 2014;71(2):870-879. doi:10.1002/mrm.24726
9. Cox RW. AFNI: Software for Analysis and Visualization of Functional Magnetic Resonance Neuroimages. Comput Biomed Res. 1996;29(3):162-173. doi:10.1006/cbmr.1996.0014
10. Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. FSL. NeuroImage. 2012;62(2):782-790. doi:10.1016/j.neuroimage.2011.09.015
11. SPM12 : Statistical Parametric Mapping package for fmri. https://www.fil.ion.ucl.ac.uk/spm/software/spm12/
12. Miller KL, Jezzard P. Modeling SSFP functional MRI contrast in the brain. Magn Reson Med. 2008;60(3):661-673. doi:10.1002/mrm.21690
13. Boxerman JL, Hamberg LM, Rosen BR, Weisskoff RM. Mr contrast due to intravascular magnetic susceptibility perturbations. Magn Reson Med. 1995;34(4):555-566. doi:10.1002/mrm.1910340412
Figures
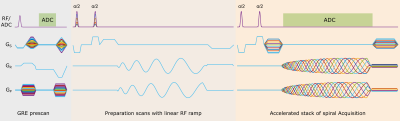
Figure 1: Sequence schematic of the spiral-out bSSFP sequence with second order water excitation: The GRE prescan with short TR(~4 ms) and 5o flip angle is used to acquire low resolution data for coil-sensitivity calibration. The preparation scan is performed for about 5-10 s to reach the steady state before the accelerated acquisition.