1249
Can MRS detect metabolites differences in fetuses affected by CMV?1Sagol School of Neuroscience, Tel Aviv University, Tel Aviv, Israel, 2Sagol Brain Institute, Tel-Aviv Sourasky Medical Center, Tel Aviv, Israel, 3School of Computer Science and Engineering, The Hebrew University of Jerusalem, Jerusalem, Israel, 4Department of Radiology, Tel-Aviv Sourasky Medical Center, Tel Aviv, Israel, 5Sackler Faculty of Medicine, Tel-Aviv University, Tel Aviv, Israel
Synopsis
Keywords: Fetal, Infection
We aimed to examine whether MR spectroscopy (MRS) within the deep grey matter can detect brain metabolic changes in fetal cytomegalovirus infection. Retrospective data from 47 fetuses with brain sonography, MRI, and MRS scans were used: 27 women had a positive PCR on amniocentesis.
Seven (out of the 27) fetuses had brain MRI findings common to CMV. NAA+NAAG, and Cr were significantly lower in fetuses with PCR+ and MRI findings. No differences were detected in fetuses without imaging findings (PCR+ and PCR-). These metabolic changes could reflect brain impairments.
Introduction
Human Cytomegalovirus (CMV) is a flu-like herpesvirus that crosses the placenta. Infection occurs in around 0.5-4% of pregnancies [1], and approximately 10-20% of newborns may develop symptoms [2]. There is an increased risk of adverse central nervous system outcomes, including hearing loss, developmental delay, and cognitive or motor function impairments [3], mainly when infection occurs in the first and second trimesters.Diagnosis of CMV includes maternal blood test and invasive amniocentesis test using PCR. MRI is used as a complementary examination to confirm ultrasound findings and detect additional anomalies not detected sonographically [1,4]. Infected fetuses may have various brain findings, including cysts, ventriculomegaly, periventricular calcifications, and white matter signal abnormalities [2,3] (Fig. 1). Termination of pregnancy may be recommended when there are severe findings. Antenatal detection is essential to ensure early intervention.
However, most fetuses affected with CMV have no imaging abnormalities in ultrasound and/or MRI and nevertheless, neonatal or delayed symptoms may be developed [2,5] and normal fetal development and outcome can’t be guarantee. Therefore, additional methods that can assess the fetus’ disease severity are necessary.
Magnetic resonance spectroscopy (MRS) provides tissue metabolic information. Several metabolites were shown to correlate with gestational age (GA) [6] and some studies suggested that MRS may show differences in metabolite’s concentrations in fetuses with CMV infection [2,7].
This study aimed to assess metabolic changes using MRS in fetuses affected by CMV, focusing on fetuses without imaging findings.
Methods
Subjects This retrospective data included fetuses scanned between 2019-2022 at our hospital with MRS data. Fetuses were referred to MRI due to various clinical indications including maternal CMV seroconversion. CMV polymerase chain reaction (PCR) data were available for the entire cohort, and fetuses were grouped accordingly to positive PCR (PCR+) or negative PCR (PCR-). Fetuses with known genetic abnormalities or data with poor imaging quality were excluded.Data acquisition Data was acquired using three 3T scanners Prisma, Vida and Skyra, (Siemens, Erlangen, Germany) and included T2 and T1 weighted images and DWI sequences, to assess brain changes. 1H-MRS was acquired using single voxel point resolved spectroscopy PRESS sequences with TR/TE = 1500/35 msec, 128 repetitions and a voxel size of 2𝑥2𝑥2 𝑐𝑚3. Region of interest (ROI) location was in deep grey matter (Fig. 2).
Prenatal imaging data, clinical and pathological information and newborn outcome at birth were collected.
Data analysis MRI was read by a senior neuroradiologist to identify brain anomalies. MRS analysis was performed using LCModel 6.3-1K, and metabolites ratios were calculated relative to Cr+PCr. (See Fig. 2). Only metabolites with SD<20% were included in the final analysis.
Statistical analysis Group differences was performed using two tailed t-test and a Benjamini-Hochberg correction for multiple comparison. A p-value<0.05 was considered statistically significant.
Results and Discussion
Subjects Figure 3 provides the subjects flow chart. A total of 193 patients were initially collected. Following MRS quality assessment (QA), 78 fetuses were excluded. This constitute of only ~60% of success in acquisition of fetal MRS data. The final cohort included 47 fetuses suspected for CMV infection due to maternal seroconversion with PCR results. 27 women had a positive PCR on amniocentesis.Imaging findings Of the 27 PCR+ fetuses, 7 had brain MRI findings typical for CMV. Using ultrasound, only two of them showed brain imaging findings. MRI and ultrasound findings in these seven fetuses are detailed in Table 1. All fetuses with PCR-, had normal imaging findings.
MRS findings PCR+ fetuses with radiology findings (N=7, mean=0.63±0.07) had a lower NAA+NAAG/Cr+PCr values compared with PCR+ fetuses without radiology findings (N=20, mean=0.8±0.11) (p = 0.02). Figure 4 shows a scatter plot of the NAA+NAAG/Cr+PCr values of the two groups. NAA is a marker of neuronal integrity and NAAG is involved in glutamate release and is in higher concentration in grey matter [8]. This result is in line with previous studies [9] using MRS in white matter, and reflect reduced tissue integrity also in the grey matter.
Preliminary results in two fetuses with PCR+ with radiology findings demonstrated a lower Cr values (mean=0.82±0.05) compared with PCR- fetuses (N=15, mean=0.98±0.04) (p = 0.002). Cr is involved in cellular energy metabolism [8].
No significant differences were detected in all metabolite values between PCR+ and PCR-.
Conclusion
We assessed MRS metabolic changes in fetuses after maternal CMV seroconversion. Only fetuses with PCR+ and radiological findings showed changes in metabolite’s values within the deep grey matter. Further studies are needed on a larger cohort to assess change in metabolites in various brain regions when MRI and ultrasound are normal.Acknowledgements
This work was supported by the Israel Innovation Authority.
References
[1] Pass, R. F., & Arav-Boger, R. (2018). Maternal and fetal cytomegalovirus infection: diagnosis, management, and prevention. F1000Research, 7.
[2] Diogo, M. C., Glatter, S., Binder, J., Kiss, H., & Prayer, D. (2020). The MRI spectrum of congenital cytomegalovirus infection. Prenatal diagnosis, 40(1), 110-124.
[3] Averill, L. W., Kandula, V. V., Akyol, Y., & Epelman, M. (2015, December). Fetal brain magnetic resonance imaging findings in congenital cytomegalovirus infection with postnatal imaging correlation. In Seminars in Ultrasound, CT and MRI (Vol. 36, No. 6, pp. 476-486). WB Saunders.
[4] Di Mascio, D., Rizzo, G., Khalil, A., D'Antonio, F., the European NeuroSOnography (ENSO) working group, Di Mascio, D., ... & D'Antonio, F. (2022). Role of fetal magnetic resonance imaging in fetuses with congenital cytomegalovirus infection: a multicenter study. Ultrasound in Obstetrics & Gynecology.
[5] Bonalumi, S., Trapanese, A., Santamaria, A., D’Emidio, L., & Mobili, L. (2011). Cytomegalovirus infection in pregnancy: review of the literature. Journal of prenatal medicine, 5(1), 1.
[6] Girard, N., Fogliarini, C., Viola, A., Confort-Gouny, S., Le Fur, Y., Viout, P., ... & Cozzone, P. (2006). MRS of normal and impaired fetal brain development. European journal of radiology, 57(2), 217-225.
[7] Mailath-Pokorny, M., Kasprian, G., Mitter, C., Schöpf, V., Nemec, U., & Prayer, D. (2012, October). Magnetic resonance methods in fetal neurology. In Seminars in Fetal and Neonatal Medicine (Vol. 17, No. 5, pp. 278-284). WB Saunders.
[8] Pugash, D., Krssak, M., Kulemann, V., & Prayer, D. (2009). Magnetic resonance spectroscopy of the fetal brain. Prenatal Diagnosis: Published in Affiliation with the International Society for Prenatal Diagnosis, 29(4), 434-441.
[9] Van der Voorn, J. P., Pouwels, P. J., Vermeulen, R. J., Barkhof, F., & Van der Knaap, M. S. (2009). Quantitative MR imaging and spectroscopy in congenital cytomegalovirus infection and periventricular leukomalacia suggests a comparable neuropathological substrate of the cerebral white matter lesions. Neuropediatrics, 40(04), 168-173.
Figures
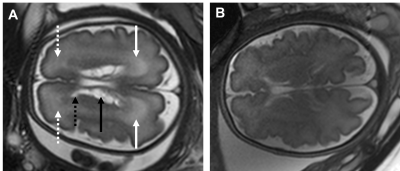
Figure 1. Fetal MRI of a 32 weeks of gestation age fetus with typical cytomegalovirus anomalies (A) and a normal brain MRI of a 32 weeks of gestation age fetus (B). White continuous and dashed arrows marks periventricular parieto-occipital and frontal T2-weighted hyperintensity. The black arrow marks intraventricular septation/pseudocysts. The dashed black arrow marks a periventricular cyst.
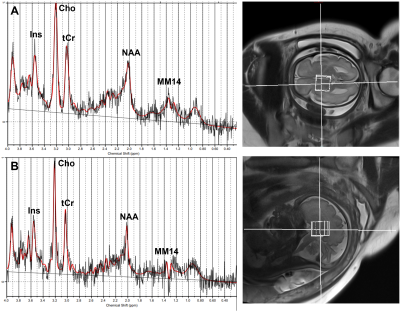
Figure 2. Example of LCModel results of spectroscopy of PCR positive fetus (A) and PCR negative fetuse (B) with deep grey matter ROI. Ins: myo-inositol. Cho: choline. tCr: total creatine. NAA: N-acetylaspartic acid. MM14: macromolecules at 1.4 ppm.
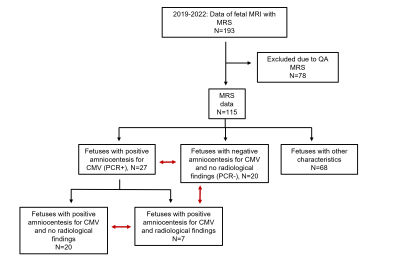
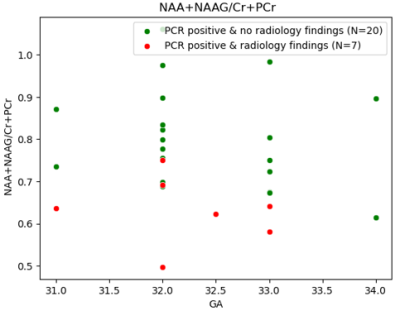
Figure 4. Scatter plot of NAA+NAAG/Cr+PCr, that was found to be significantly lower in the subgroup of PCR+ with radiology findings (in red, N=7, mean=0.63±0.07) compared to the subgroup of PCR+ without radiology findings (in green , N=20, mean=0.8±0.11) (p = 0.02).
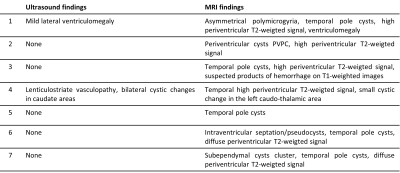
Table 1. Ultrasound and MRI brain findings for the subgroup of PCR positive fetuses with radiologic findings (N=7).