1247
Motion-corrected free-running 4D MRI of the fetal heart - from in silico to in vivo1Department of Radiology, Lausanne University Hospital (CHUV) and University of Lausanne (UNIL), Lausanne, Switzerland, 2Advanced Cardiothoracic Imaging Unit, Department of Imaging, Bambino Gesù Children’s Hospital IRCCS, Rome, Italy, 3Siemens Healthcare srl, Milan, Italy, Milan, Italy, 4Department of Radiology and Interventional Radiology, Lausanne University Hospital (CHUV) and University of Lausanne (UNIL), Lausanne, Switzerland, 5Woman- Mother-Child Department, Lausanne University Hospital (CHUV) and University of Lausanne (UNIL), Lausanne, Switzerland, 6Advanced Clinical Imaging Technology (ACIT), Siemens Healthineers International AG, Lausanne, Switzerland, 7LTS5, École Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland, 8Center for Biomedical Imaging (CIBM), Lausanne, Switzerland
Synopsis
Keywords: Fetal, Cardiovascular
MR imaging of the fetal heart is challenging due to resolution requirements and the impact of maternal respiration, fetal cardiac motion, and gross fetal movement. These factors have largely precluded the development of 3D acquisition techniques. In this work, a novel reconstruction algorithm is developed to estimate and correct for displacement of the fetal heart due to maternal respiration and gross fetal movement enabling the first-ever motion-corrected time-resolved 4D images of the fetal heart from 3D radial data. Proof-of-concept results are demonstrated using a comprehensive numerical simulation developed for this work and initial data acquired in utero.Introduction
Technical developments have driven the use of MRI to complement ultrasound in the evaluation of the fetal heart, providing new ways to manage cardiovascular diseases detected in utero 1. Fetal cardiac MRI acquisitions are designed with the shortest scan times possible to avoid artifacts and blur from maternal respiratory motion, fetal cardiac motion, and gross fetal movement. A balance must therefore be struck between the need for abbreviated scan times, the spatial resolution necessary to visualize the small vessels and chambers of the fetal heart, the temporal resolution needed to resolve the fast fetal heart rate, and the volumetric coverage required to interrogate the complex 3D cardiac anatomy. As a result, a series of 2D images combined with motion-correction, scattered data interpolation, and super-resolution algorithms 2,3 provide a surrogate for dynamic 3D (4D) MRI evaluation of the fetal heart. However, 2D acquisitions may be limited by through-plane motion and have a constrained spatial resolution in the slice selection direction. Alternatively, the use of 3D acquisitions has recently been proposed, greatly simplifying scan planning 4,5, but no methods for motion-compensation have been published to date. In this work, we therefore propose a novel reconstruction method for 4D MRI of the fetal heart using a continuous 3D radial acquisition with isotropic spatial resolution. Our proposed reconstruction algorithm retrospectively identifies and corrects both displacement of the fetal heart due to maternal respiration as well as gross fetal movement. To validate our approach and to inform in utero parameter ranges, a complex numerical simulation framework is developed providing a necessary but not yet available ground truth for developing 3D fetal imaging techniques. Here, we present our initial findings using our numerical phantom and demonstrate the first ever motion-corrected 4D images of the fetal heart from 3D radial data acquired in utero.Methods
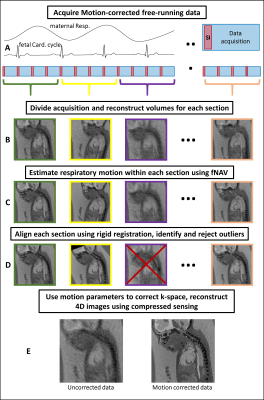
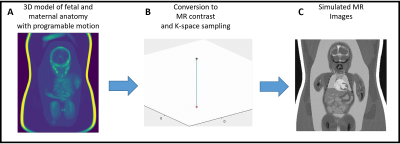
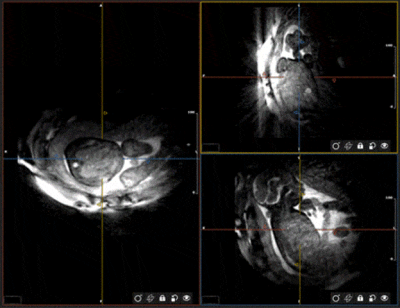
Fig. 1 outlines the proposed algorithm for estimating displacement of the fetal heart due to maternal respiration 6 and gross fetal movement by exploiting intermediate “real-time” reconstructions of 3D radial data. The motion estimates are used to correct the k-space data and reject outliers 2. The remaining data are retrospectively binned according to their cardiac phase and reconstructed as 4D images 8. To characterize this algorithm, we designed a comprehensive numerical simulation of the maternal and fetal anatomy (Fig. 2) 7. It includes 3D programmable motion (respiration and bulk movement), MR contrast, and 3D k-space sampling schemes. In silico data sets (N=50) were generated with maximum 3D translational motion amplitudes of 10 mm, maximum 3D rotation of 5°, and scan parameters matching in utero data. One pregnant volunteer who gave consent (32 weeks gestational age) was scanned using a previously described free-running 3D radial bSSFP research sequence 8 without fat suppression or ramp-up pulses, on a 1.5T clinical MRI scanner (MAGNETOM Aera, Siemens Healthcare, Erlangen, Germany). For in silico data, motion-corrected, and uncorrected images were compared and the image blur relative to the ground truth was measured 9. For in utero data retrospective binning into 20 cardiac phases was performed using an MRI-compatible Doppler ultrasound gating device 10 and compressed sensing reconstruction was performed using spatial and temporal regularization weights of 0.001 and 0.05 respectively. 4D in utero images were visually inspected using Circle (cvi42, Circle Cardiovascular Imaging, Calgary, Canada).Results
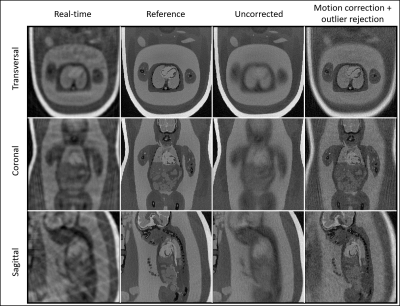
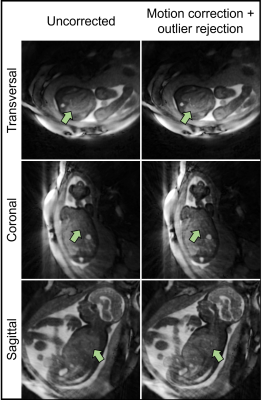
Bulk fetal movement is well visualized by real-time reconstructions (Fig. 3a) of the in silico data, as shown in transversal, sagittal, and coronal orientations. When compared to the motion-free reference (Fig. 3b), this movement leads to significant blur in the uncorrected images. Conversely, the motion-corrected images recover fine details of the heart despite the underlying maternal respiration and gross fetal movement. Quantitative measurement of image blur corroborates this visual result with corrected images yielding low amounts of blur (0.5±0.3) relative to the uncorrected image (3.3±0.1). Fig. 4 provides a comparison between uncorrected and motion-corrected images obtained in utero. A clear improvement in image quality and delineation of the fetal cardiac anatomy is obtained using motion-correction corroborating the in silico result. However, streaking artifacts originating from the high maternal fat signal remains. Finally, Fig. 5 provides an animation of the final 4D image reconstructions from in utero data highlighting the ability to retrospectively interrogate the dynamic anatomy of the fetal heart in arbitrary scan planes due to the 3D isotropic spatial resolution and large volumetric coverage achieved by this technique.Discussion and Conclusion
A novel algorithm for motion-corrected dynamic volumetric imaging of the fetal heart was developed. Its initial use was investigated using a numerical simulation and its feasibility was demonstrated in utero providing the first ever motion-corrected 4D images of the fetal heart from a continuous 3D radial acquisition. Further investigation is required to determine the degree of motion that can be accurately corrected. Additionally, improvements to the acquisition are needed to reduce artifacts unrelated to motion. Nevertheless, the numerical simulation provided by this work already creates a tool for exploring the impact of motion, as well as future optimizations. This is all in keeping with the goal of providing a high-resolution volumetric assessment of the fetal heart for continued improvement in our ability to manage cardiovascular disease discovered in utero.Acknowledgements
MS is the PI on the Swiss National Science Foundation grants 320030_173129 and 201292 that funded part of this research. CWR is the PI on Swiss National Science Foundation Grant PZ00P3_202140 that funded part of this research.References
1. Roy CW, van Amerom JFP, Marini D, Seed M, Macgowan CK. Fetal Cardiac MRI: A Review of Technical Advancements. Top Magn Reson Imaging. 2019 Oct;28(5):235-244.
2. van Amerom JFP, Lloyd DFA, Deprez M, Price AN, Malik SJ, Pushparajah K, van Poppel MPM, Rutherford MA, Razavi R, Hajnal JV. Fetal whole-heart 4D imaging using motion-corrected multi-planar real-time MRI. Magnetic Resonance in Medicine. 2019 May;82(3):1055-1072.
3. Roberts TA, van Amerom JFP, Uus A, Lloyd DFA, van Poppel MPM, Price AN, Tournier JD, Mohanadass CA, Jackson LH, Malik SJ, Pushparajah K, Rutherford MA, Razavi R, Deprez M, Hajnal JV. Fetal whole heart blood flow imaging using 4D cine MRI. Nature Communications. 2020 Oct;11(4992).
4. Piek M, Ryd D, Töger J, Testud F, Hedström E, Aletras AH. Fetal 3D cardiovascular cine image acquisition using radial sampling and compressed sensing. Magn Reson Med. 2022 Sep;1-11.
5. Knapp J, Tavares de Sousa M, Lenz A, Herrmann J, Zhang S, Kording F, Hergert B, Adam G, Bannas P, Schoennagel BP. Fetal 4D flow MRI of the great thoracic vessels at 3 Tesla using Doppler-ultrasound gating: a feasibility study. Eur Radiol. 2022 Oct.
6. Roy CW, Heerfordt J, Piccini D, Rossi G, Pavon AG, Schwitter J, Stuber M. Motion compensated whole-heart coronary cardiovascular magnetic resonance angiography using focused navigation (fNAV). J Cardiovasc Magn Reson. 2021 Mar;23(1):33.
7. Roy CW, Marini D, Segars WP et al. Fetal XCMR: a numerical phantom for fetal cardiovascular magnetic resonance imaging. J Cardiovasc Magn Reson. 2019 May;21(29).
8. Di Sopra L, Piccini D, Coppo S, Stuber M, Yerly J. An automated approach to fully self-gated free-running cardiac and respiratory motion-resolved 5D whole-heart MRI. Magn Reson Med. 2019 Dec;82(6):2118-2132.
9. Roy CW, Seed M, Kingdom JC, Macgowan CK. Motion compensated cine CMR of the fetal heart using radial undersampling and compressed sensing. J Cardiovasc Magn Reson. 2017 Mar;19(29).
10. Kording F, Yamamura J, de Sousa MT, Ruprecht C, Hedström E, Aletras AH, Ellen Grant P, Powell AJ, Fehrs K, Adam G, Kooijman H, Schoennagel BP. Dynamic fetal cardiovascular magnetic resonance imaging using Doppler ultrasound gating. J Cardiovasc Magn Reson. 2018 Mar;20(1):17.
Figures