1201
Neurovascular decoupling in type 2 diabetes: A meta-analysis and an independent validation of Neuroimaging1Hospital, Fourth Military Medical University, 569 Xinsi Road, Xi’an 710038, Shaanxi, China, Xi’an, China
Synopsis
Keywords: Nerves, fMRI (resting state)
Disrupted neurovascular (NV) coupling is considered as a potential mechanism of Type 2 diabetes mellitus (T2DM) induced mild cognitive impairment (MCI). The study quantitatively explored whether NV decoupling were associated with cognitive impairment in patients with T2DM by means of neuroimaging meta-analysis and an independent validation. In T2DM, NV uncoupling existed in many brain regions, and the degree of uncoupling was related to cognition. It contributed to a better understanding of the mechanism in T2DM cognitive impairment and would be a promising neuroimaging biomarker. This study combined meta-analysis and independent validation model can be extended to other similar studies.Introduction
Type 2 diabetes mellitus (T2DM) is a systemic disease characterized by elevated blood glucose and insulin resistance[1]. T2DM often causes a variety of complications [2]. Nearly half of patients with T2DM suffer from mild cognitive impairment (MCI)[3], which seriously affects the life quality of patients[4]. More and more researchers pay attention to T2DM related brain alteration. The mechanism of T2DM induced MCI need to be explored urgently. Neuroimaging especially functional magnetic resonance imaging (fMRI) provides a method for studying brain abnormality, which is promising for identifying MCI in the early stage of T2DM and preventing it progress to dementia[5].Combined rs-fMRI and ASL, some neuroimaging studies further showed that T2DM was accompanied with NV decoupling, which played an important role in the occurrence of MCI[6-8]. Our team previously found that there were NV decoupling regions associated with cognitive impairment in T2DM patients, and put forward that mean CBF (mCBF)-ALFF, mCBF-DC and mCBF-ReHo could be considered as potential biological markers of early T2DM-MCI[7]. Another five-year follow-up study also found that T2DM might accelerate NV decoupling impairment in the left insula, leading to the decline of memory[8]. However, only a few of studies explored the NV decoupling of T2DM, and there were still problems of small sample size, inconsistency and poor repeatability in NV neuroimaging studies. Thus, there was an urgent need to conduct a reliable large sample study on the NV decoupling in T2DM patients by means of meta-analysis and to validate NV decoupling features in our own data set.
Methods
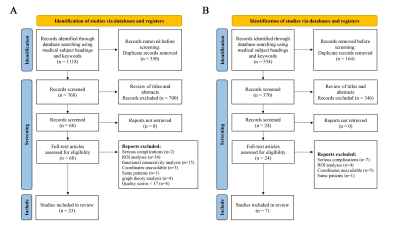
The current study was divided into two parts: meta-analysis and independent validation. The data extracted for meta-analysis included coordinates, t value and sample size extracted from published fMRI papers of T2DM patients. The data extracted for independent validation included the rs-fMRI and ASL images from 35 T2DM patients and 30 healthy controls (HCs).Neuroimaging meta-analysis The meta-analysis was performed according to the standards of Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) (See Supplementary Material) and ten simple rules for neuroimaging meta-analysis[9,10]. The protocol for this neuroimaging meta-analysis was registered on PROSPERO (CRD42022330674) (https://www.crd.york.ac.uk/prospero/).
35 T2DM patients and 30 HCs were enrolled in the validation study. The diagnosis of T2DM was based on the latest standards of the American Diabetes Association [59]. All the participants were between 35 and 70 years old. Subjects who met any of the exclusion criteria were excluded: (1) foreign metal implant in or around their body; (2) pregnant or claustrophobic subjects; (3) history of serious brain diseases (major brain trauma, tumor, stroke, meningitis, cerebral infarction) or myocardial infarction; (4) central nervous system diseases, or internal medicine diseases that had a significant impact on neurological function; (5) individuals taking psychoactive or steroid drugs within 3 months; (6) poor image quality or excessive head movement (translation > 3.0 mm or rotation > 3°); (7) diabetes with a serious complication. Three T2DM patients were excluded because of excessive head movement. Two T2DM patients were excluded because of severe complications including encephalomalacia and cerebral infarction. One HC subject was excluded because of poor spatial registration. Finally, a total of 30 T2DM patients and 29 age- and sex- matched HCs were recruited for the present study.
Results
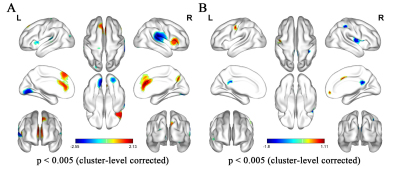
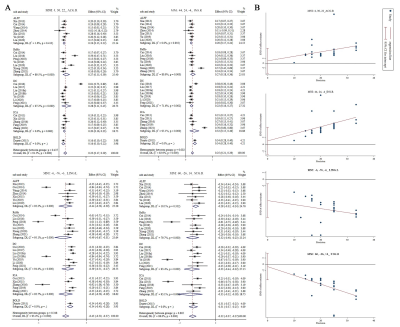
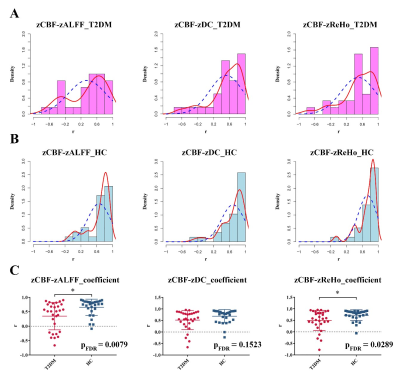
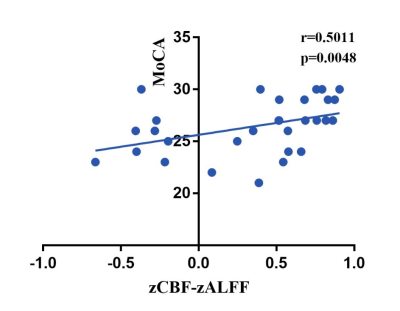
In the current meta-analysis, there was neurovascular decoupling in the left lingual gyrus, right superior temporal gyrus, bilateral cingulate gyrus and right insula in T2DM. The results of fMRI independent validation datasets showed that the coupling coefficients of zCBF-zALFF and zCBF-zReHo decreased in the left lingual gyrus and right superior temporal gyrus in T2DM compared with HCs. The coupling coefficient of zCBF-zALFF in the left lingual gyrus of T2DM patients was related to cognition, which suggested that the NV coupling coefficients were more sensitive to cognitive impairment in the T2DM patients. Our results contributed to a better understanding of the mechanism in T2DM cognitive impairment and would be a promising neuroimaging biomarker.Discussion
Our fMRI datasets validated some of the results of our meta-analysis. On the one hand, this showed that T2DM might lead to MCI through NV decoupling, which damaged the working memory ability of T2DM patients, especially the visuospatial memory ability. The coupling of zCBF-zALFF, zCBF-zDC and zCBF-zReHo were used as indicators to reflect the state of NV decoupling and explained T2DM related cognitive impairment. On the other hand, the results of small sample neuroimaging studies might only be applicable to specific populations, reflecting the uncoupling pattern of neurovascular systems in specific populations. Our meta-analysis and independent validation showed that neuroimaging research should more strictly control the data quality and verify the reliability of the results from multiple angles and directions.In the early stage of T2DM, NV uncoupling existed in many brain regions, and the degree of uncoupling was related to cognition. NV coupling coefficient is more sensitive to cognitive impairment in early stage of T2DM. It contributes to a better understanding of the mechanism in T2DM cognitive impairment and would be a promising neuroimaging biomarker. This study combined meta-analysis and independent validation model can be extended to other similar studies.
Acknowledgements
No acknowledgement found.References
1. Krentz NAJ, Gloyn AL. Insights into pancreatic islet cell dysfunction from type 2 diabetes mellitus genetics. Nature reviews Endocrinology 2020;16(4):202-12. doi: 10.1038/s41574-020-0325-0.
2. Magliano DJ, Sacre JW, Harding JL, Gregg EW, Zimmet PZ, Shaw JE. Young-onset type 2 diabetes mellitus - implications for morbidity and mortality. Nature reviews Endocrinology 2020;16(6):321-31. doi: 10.1038/s41574-020-0334-z.
3. You Y, Liu Z, Chen Y, Xu Y, Qin J, Guo S, et al. The prevalence of mild cognitive impairment in type 2 diabetes mellitus patients: a systematic review and meta-analysis. Acta diabetologica 2021;58(6):671-85. doi: 10.1007/s00592-020-01648-9.
4. Biessels GJ, Strachan MW, Visseren FL, Kappelle LJ, Whitmer RA. Dementia and cognitive decline in type 2 diabetes and prediabetic stages: towards targeted interventions. The lancet Diabetes & endocrinology 2014;2(3):246-55. doi: 10.1016/s2213-8587(13)70088-3.
5. Rosenberg J, Lechea N, Pentang GN, Shah NJ. What magnetic resonance imaging reveals - A systematic review of the relationship between type II diabetes and associated brain distortions of structure and cognitive functioning. Frontiers in neuroendocrinology 2019;52:79-112. doi: 10.1016/j.yfrne.2018.10.001.
6. Hu B, Yan LF, Sun Q, Yu Y, Zhang J, Dai YJ, et al. Disturbed neurovascular coupling in type 2 diabetes mellitus patients: Evidence from a comprehensive fMRI analysis. NeuroImage Clinical 2019;22:101802. doi: 10.1016/j.nicl.2019.101802.
7. Yu Y, Yan LF, Sun Q, Hu B, Zhang J, Yang Y, et al. Neurovascular decoupling in type 2 diabetes mellitus without mild cognitive impairment: Potential biomarker for early cognitive impairment. NeuroImage 2019;200:644-58. doi: 10.1016/j.neuroimage.2019.06.058.
8. Zhang Y, Zhang X, Ma G, Qin W, Yang J, Lin J, Zhang Q. Neurovascular coupling alterations in type 2 diabetes: a 5-year longitudinal MRI study. BMJ open diabetes research & care 2021;9(1) doi: 10.1136/bmjdrc-2020-001433.
9. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (Clinical research ed) 2021;372:n71. doi: 10.1136/bmj.n71.
10.1136/bmj.n71. 10. Müller VI, Cieslik EC, Laird AR, Fox PT, Radua J, Mataix-Cols D, et al. Ten simple rules for neuroimaging meta-analysis. Neuroscience and biobehavioral reviews 2018;84:151-61. doi: 10.1016/j.neubiorev.2017.11.012.
Figures