1197
Associations Between Central Canal Stenosis, Resting State Functional Connectivity Networks, and Pain Perception
Jennifer Anne Cummings1, Madeline Hess1, Kenneth Gao1, Upasana Bharadwaj1, Misung Han1, Cynthia Chin1, Salvatore Torrisi1, Jennifer Townsend1, An Vu1, Valentina Pedoia1, and Sharmila Majumdar1
1Radiology and Biomedical Imaging, University of California, San Francisco, San Francisco, CA, United States
1Radiology and Biomedical Imaging, University of California, San Francisco, San Francisco, CA, United States
Synopsis
Keywords: Nerves, Nervous system
Chronic lower back pain remains difficult to characterize with imaging. With this study, we investigate the relationship between imaging-based brain and spine biomarkers in pain perception. We present relationships between resting state functional connectivity networks, automatic spinal canal stenosis grading, and patient-reported pain measures. Patients reporting severe pain show weaker connectivity in the Left Ventral Attention Network and stronger connectivity within the Salience network when compared to those reporting no pain or mild/moderate pain. The severe back pain group also shows a higher spinal canal stenosis grading.Background
Chronic lower back pain (cLBP) is the leading cause of disability and loss of quality of life in the US1. Several factors make back pain particularly difficult to characterize, including the diversity of tissues implicated, lack of standardized quantification methods, and the inherent subjectivity of sensation. While brain fMRI holds the possibility of revealing the neural activity underlying pain sensation2, a comprehensive understanding of pain must also take peripheral inputs into account. In this study, we present preliminary findings from a prospective study in which both spine and brain MRI data are collected from the same subjects over multiple time points. Leveraging recent developments in machine learning, we obtain a measure of central canal stenosis in the spine for each subject as well as connectivity strength within functional networks in the brain during resting state fMRI. We present relationships between these spine and brain biomarkers with common pain metrics. In doing so, we take the first steps in establishing an imaging-based holistic model of pain.Methods
Image Acquisition: Brain and spine images were collected for 10 cLBP patients and 3 healthy controls (13 unique subjects; age = 56.8 ± 10.8, BMI = 29.0 ± 7.1, 11 Female). 7 patients also completed a 12-week follow-up visit, amounting to a total of 20 samples for this study. All images were collected at UCSF on a 3T GE SIGNA scanner. Spine imaging protocol included the acquisition of axial T2-weighted fast spin echo sequences. Neuroimaging protocol followed standards set by the Human Connectome Project and included 3D T1-weighted MP-RAGE and resting state fMRI using multiband echo planar imaging. Questionnaires to assess pain and functioning, including the Visual Analog Scale (VAS) and Oswestry Disability Index (ODI), were also administered at each visit.Spine image processing: Automatic segmentation of the dural sac and intervertebral discs was performed using convolutional neural networks (CNN’s) based on 2D V-Nets trained on axial T2-weighted images. Models have been previously published and performances are summarized in Figure 13. Segmentation masks were used to calculate dural sac and disc cross-sectional area (CSA) at each axial slice. The ratio of the dural sac and disc CSA’s (DDR) was used as input into a decision tree classifier trained to classify each axial slice with a grade of central canal stenosis (Normal, Mild, Moderate, Severe) based on using a method previously described4. A summary statistic was obtained for each subject by calculating the sum of the weighted value counts of each stenosis grade for the entire axial volume (“DDR score”).
Neuroimaging Processing: Processing was performed using fMRIPrep 21.0.25 and Nilearn6. Skull-stripped T1-weighted images were used for brain tissue segmentation and registration into standard MNI space. Functional data processing included slice-timing correction, alignment to T1w reference, removal of motion artifacts and global confounds, and band-pass filtering. Parcellation of time series into probabilistic functional networks was performed using the Multi-subject dictionary learning (MSDL) Atlas7. Pearson’s correlation coefficient was applied to the parcellated time series to obtain functional connectivity strength within 11 resting state functional networks (Auditory, Default Mode, Right ventral attention, Left ventral attention, Dorsal attention, Visual, Salience, Temporal, Language, Cingulate-Insula, Anterior intraparietal sulcus). Fisher’s z transform was applied to functional connectivity strengths to produce a normal distribution.
Statistical analysis: Linear regression was performed to test for association between central canal stenosis metrics and brain functional biomarkers against each of 3 outcome variables of interest (VAS “Back Pain” [0 - 10], ODI “Pain Intensity” [0 - 5], ODI overall score [0 - 100%]). Correlations with a corresponding p-value less than 0.05 are reported. Partial correlation was used to control for confounding variables of age, sex, and BMI. Subjects were also divided into groups using a threshold of VAS pain score greater than or less than 6, a previously documented metric for severe pain8. Differences in spine and pain biomarkers were assessed between the two groups using the two-sample t-test.
Results
Connectivity strength within the Left Ventral Attention Network was correlated with both overall ODI score (p=0.0011; adjusted p=0.0019, CI=[-0.88, -0.33]) and ODI Back Pain subscore (p=0.010; adjusted p=0.010, CI=[-0.84, -0.18]). Initial regression indicated a significant correlation between VAS back pain and DDR score (p=0.012). After controlling for confounding variables, this relationship approaches significance with (p adjusted= 0.056, CI=[-0.01, 0.78]). When subjects were divided into high and low VAS groups, differences were shown between the connectivity strengths in both the Left Ventral Attention Network (p=0.0095, T-stat=-2.90) and the Salience Network (p=0.023, T-stat=2.50) as well as between DDR scores (p=0.040, T-stat=2.22).Conclusions
Preliminary results indicate a negative correlation between functional connectivity strength in the Left Ventral Attention Network at rest and pain perception. Although more data is needed, this relationship may be indicative of attention divergence to pain as opposed to other external stimuli9. Results also indicate the potential utility of an automatically quantified metric of central canal stenosis in predicting reported pain measures.Acknowledgements
This research was supported by NIH UH3AR076724References
- Hoy, Damian, Lyn March, Peter Brooks, Anthony Woolf, Fiona Blyth, Theo Vos, and Rachelle Buchbinder. "Measuring the global burden of low back pain." Best practice & research Clinical rheumatology 24, no. 2 (2010): 155-165.
- Mouraux, André, and Gian Domenico Iannetti. "The search for pain biomarkers in the human brain." Brain 141, no. 12 (2018): 3290-3307.
- Bharadwaj, U.U. et al. Deep learning for automated, interpretable classification of lumbar spinal stenosis and facet arthropathy from axial MRI. European Radiology. In review.
- Bharadwaj, U. U., A. R. Ben-Natan, J. Huang, V. Pedoia, D. Chou, S. Majumdar, T. M. Link, and C. T. Chin. "Evaluation of 2 Novel Ratio-Based Metrics for Lumbar Spinal Stenosis." American Journal of Neuroradiology 43, no. 10 (2022): 1530-1538.
- Esteban, Oscar, Christopher J. Markiewicz, Ross W. Blair, Craig A. Moodie, A. Ilkay Isik, Asier Erramuzpe, James D. Kent et al. "fMRIPrep: a robust preprocessing pipeline for functional MRI." Nature methods 16, no. 1 (2019): 111-116.
- Gorgolewski, Krzysztof, Christopher D. Burns, Cindee Madison, Dav Clark, Yaroslav O. Halchenko, Michael L. Waskom, and Satrajit S. Ghosh. "Nipype: a flexible, lightweight and extensible neuroimaging data processing framework in python." Frontiers in neuroinformatics (2011): 13.
- Gael Varoquaux, Alexandre Gramfort, Fabian Pedregosa, Vincent Michel, and Bertrand Thirion. Multi-subject dictionary learning to segment an atlas of brain spontaneous activity. In Information Processing in Medical Imaging, 562–573. Berlin, Heidelberg, 2011. Springer Berlin Heidelberg.
- Boonstra, Anne M., Henrica R. Schiphorst Preuper, Gerlof A. Balk, and Roy E. Stewart. "Cut-off points for mild, moderate, and severe pain on the visual analogue scale for pain in patients with chronic musculoskeletal pain." Pain® 155, no. 12 (2014): 2545-2550.
- Vossel, Simone, Joy J. Geng, and Gereon R. Fink. "Dorsal and ventral attention systems: distinct neural circuits but collaborative roles." The Neuroscientist 20, no. 2 (2014): 150-159.
Figures
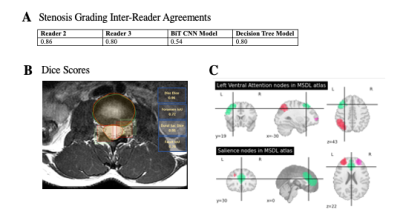
A)
Inter-reader agreements between between Reader 1 (neuroradiologist) and Reader
2 (radiology trainee), Reader 3 (musculoskeletal radiologist), big transfer (BiT)
convolutional neural network (CNN) model, and decision tree model to classify
stenosis. B) Example results of segmentation model, with bounding boxes (red)
compared to ground-truth (green) for the right facet and left foramen. C)
Regions included in brain networks of interest
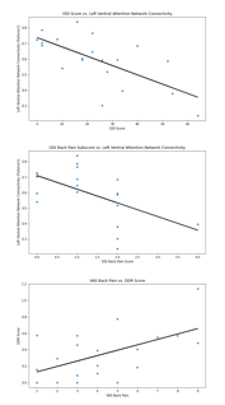
Scatter plots showing correlation between biomarkers and pain outcome variables

Box plots of biomarkers that vary significantly between subjects who report severe back pain vs low-to-moderate pain
DOI: https://doi.org/10.58530/2023/1197