1195
Altered white matter diffusion properties and gray matter volume in classical trigeminal neuralgia
Yang Zhang1, Wei Su2, Xiaoyu Du3, Rui Li4, Hang Zhao4, Zhaoping Wang1, Lei Feng1, liangjie lin5, and Kaihua Zhang2
1Department of Neurosurgery, Jining No. 1 People’s Hospital, Jining, China, 2School of Psychology, Shandong Normal University, Ji’nan, China, 3Faculty of Medicine, Dentistry and Health Sciences, The University of Melbourne, Victoria, Australia, 4Department of Radiology, Jining No. 1 People’s Hospital, Jining, China, 5Clinical and Technical Support, Philips Healthcare, Beijing, China
1Department of Neurosurgery, Jining No. 1 People’s Hospital, Jining, China, 2School of Psychology, Shandong Normal University, Ji’nan, China, 3Faculty of Medicine, Dentistry and Health Sciences, The University of Melbourne, Victoria, Australia, 4Department of Radiology, Jining No. 1 People’s Hospital, Jining, China, 5Clinical and Technical Support, Philips Healthcare, Beijing, China
Synopsis
Keywords: Nerves, Diffusion Tensor Imaging, Trigeminal neuralgia, trigeminal nerve fiber
Investigating microstructural changes in patients with classical trigeminal neuralgia (CTN) has contributed to understanding the pathological neural mechanism of CTN. This study aimed to reveal abnormalities of the trigeminal fiber bundles by combing MR diffusion tensor imaging (DTI) with voxel-based morphometry (VBM). 32 patients with CTN and 32 matched healthy controls were recruited with the main fiber bundle diffusion indices calculated and the whole-brain gray matter volume measured for each subject. Results showed that CTN exhibited microstructural changes in the trigeminal nerve fibers, and the changes might be associated with the pathogenesis of trigeminal neuralgia.Introduction
Classical trigeminal neuralgia (CTN) is a rare but excruciatingly painful condition, and fundamental questions related to its cause, prevalence, and underlying mechanism remain unanswered. CTN is thought to be caused by neurovascular compression of the trigeminal nerve fibers at its root entry zone. However, it is unknown whether microstructural changes occur in the trigeminal nerve fibers in patients with CTN. Thus, MR diffusion tensor imaging (DTI) was used in the current study to reveal abnormalities of the trigeminal fiber bundles. Additionally, we quantified brain structural abnormalities present in CTN patients to define the possible pathogenetic mechanism of this disease.Materials and Methods
32 clinical CTN patients and 32 matched healthy controls (HCs) were recruited in this study with demographic and clinical data collected, including age, sex, disease duration, side affected, presence or absence of neurovascular compression, atrophy, and pain intensity of migraine attacks measured by visual analog scale (VAS) (See Table 1). The study traced homogeneous fiber bundles of the trigeminal system, classified them by anatomical origin, and compared the diffusion indices of these fiber tracts. Voxel-based morphometry (VBM) was used to quantitatively investigate the whole-brain gray matter (GM) distribution. All subjects underwent MRI scans on a 3.0 T scanner (Trio system; Siemens; Germany) with a 12-channel head coil using a standardized protocol. The 3D T1-weighted imaging parameters were as follows: repetition time (TR)=1900 ms; echo time (TE)=2.52 ms; field of view (FOV)=256 mm×256 mm; voxel dimension=1 mm×1 mm×1 mm; 176 continuous axial direction slices, slice thickness of 1 mm with no gaps; The diffusion tensor imaging was acquired with 30 noncollinear directions (b=1000 s/mm2) together with an acquisition without diffusion weighting (b=0 s/mm2); TR=10000 ms; TE=91 ms; FOV=256 mm×256 mm; voxel dimension=2 mm×2 mm×2 mm. The study traced homogeneous fiber bundles of the trigeminal system, classified them by anatomical origin, and compared the diffusion indices of these fiber tracts. Voxel-based morphometry (VBM) was used to quantitatively investigate the whole-brain gray matter (GM) distribution.Results
There were no significant differences in age or sex between the CTN patients and HCs (P > 0.05). All patients had long-standing right-sided TN symptoms (mean duration 7.0 ± 2.6 years). The average pain severity on a visual analog scale (VAS) was 6.6 ± 2.3. VBM results revealed decreases in the GM volume of several areas in CTN patients compared to HCs, these regions included the ones involved in pain processing (e.g., the frontal cortex) and emotional, cognitive, and autonomic functions (e.g., the cingulate cortex, precuneus, amygdala, and parahippocampal gyrus) (Figure 1). DTI analysis indicated that significantly lower fractional anisotropy (FA) values in the region of the spinal trigeminal tract in CTN patients than in HCs (P = 0.008), where the mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD) were significantly higher in CTN patients than in HCs (MD: P = 0.010; AD: P = 0.011; RD: P =0.022) . Correlation analyses revealed FA showed a significant negative correlation with VAS scores in the CTN group (r = -0.461, P = 0.008), while RD showed a significant positive correlation with VAS scores (r = 0.351, P = 0.049) (Figure 2).Discussion
We found decreased FA in the trigeminal nerve tracts, which may contribute to the dysfunction of trigeminal nociception and multimodal information integration in CTN. CTN was associated with reduced GM volume and increased AD, RD, and MD in the trigeminal nerve tracts, which further strengthens the evidence linking CTN to the trigeminal nerve tracts. Reduced GM volume in several regions, especially in cortical regions implicated in the multidimensional experience of pain. These regions included areas are involved in pain processing (e.g., the frontal cortex), as well as those associated with emotional, cognitive, and autonomic functions (e.g., the cingulate cortex, precuneus, amygdala, and parahippocampal gyrus)1,2, that could contribute to brain dysfunction in the transmission and modulation of noxious information from the cranial vessels and meninges.Conclusion
CTN patients were observed to exhibit microstructural changes in the trigeminal nerve fibers. Abnormalities of GM structural networks in certain brain regions were found in the CTN group compared with the HC group, suggesting that the pathophysiology of CTN might correspond to alterations in these regions. Microstructural changes in trigeminal fibers in the pons are implicated in the pathogenesis of trigeminal neuralgia. The accurate localization of abnormal trigeminal fiber bundles in the pons provides insight into the pathogenic mechanism of trigeminal neuralgia and guidance for the rational selection of surgical methods.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant Nos. 32200915). We would like to acknowledge the dedication of all the participants involved in this research.References
1. Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia 38: 1-211, 2018.
2. Zhang Y, Dong S, Fang W, Chai X, Mei J, and Fan X. Self-efficacy for self-regulation and fear of failure as mediators between self-esteem and academic procrastination among undergraduates in health professions. Adv Health Sci Educ Theory Pract 23: 817-830, 2018.
Figures
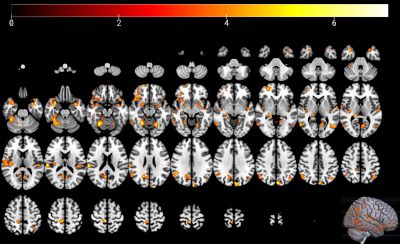
Figure 1. Brain regions with
significant differential GM volume between CTN and HCs.
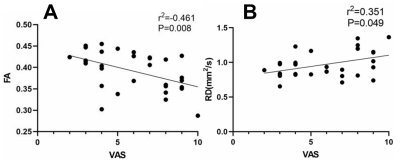
Figure 2. Fiber bundle diffusion
index with significant correlated to VAS scores in CTN patients. (A)
associations between the FA values and VAS scores in CTN patients; (B)
associations between the RD values and VAS scores in CTN patients.
DOI: https://doi.org/10.58530/2023/1195