1193
Pathogenesis of Chiari Malformation:insights from morphology,neurobiology and hydrodynamics based on MR neuroimaging technology1Radiology, Sichuan Provincial People's Hospital, Chengdu, China, 2Department of Neurosurgery, Sichuan Provincial People's Hospital, Chengdu, China, 3MR Scientific Marketing, Siemens Healthcare, Shanghai, China
Synopsis
Keywords: Nerves, Nerves
The pathogenic mechanism of different clinical and radiologic manifestations of Chiari malformation I (CMI) is still unclear. Using MR morphology, neurobiology, and hydrodynamics techniques allow for a comprehensive assessment of CMI. FA values and CSF flow may well explain the correlation between white matter fiber tract, CMI pain, and compensations at other body parts. MRI technology helps us to better understand the pathogenic mechanism of CMI and can provide more valuable information in the diagnosis and treatment of CMI.Introduction
Chiari malformation I(CMI)can be defined as position of the cerebellar tonsils 5 mm or more below the foramen magnum(FM)and clinical presentation can vary considerably,from clinical silence to debilitating headaches or life-threatening bulbar abnormalities.Neurologic symptoms result from 3 pathologic mechanisms:obstruction of the cerebrospinal fluid(CSF)flow,brainstem compression,and syringomyelia.The pathogenic mechanism of different types of CMI including symptomatic CMI without tonsillar herniation(CM0),asymptomatic CMI(ACMI),symptomatic CMI(SCMI) is unclear.Morphometric measures of CMI including extent of tonsillar herniation can be performed using mprage squence1.DTI might provide a valuable insight into the neurobiological foundation of SCMI presentation 2-5.By phase-contrast magnetic resonance imaging(PC-MRI),impaired CSF flow pulsation below the foramen magnum during systole can be observed, as well as improved CSF flow after posterior fossa decompression or surgical suboccipital craniectomy.The aim of this study was to explore the pathogenic mechanism of different types of CMI from MR morphology,neurobiology,and hydrodynamics.Methods
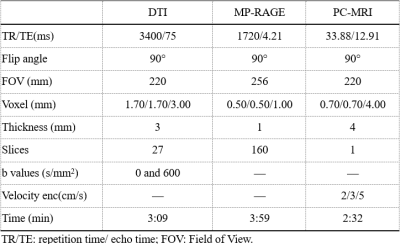
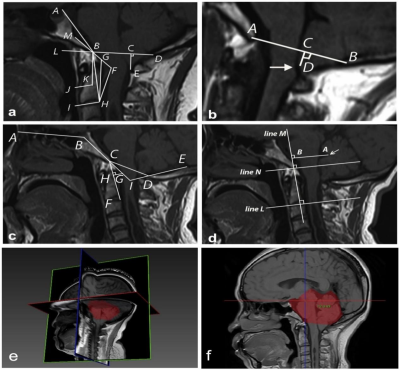
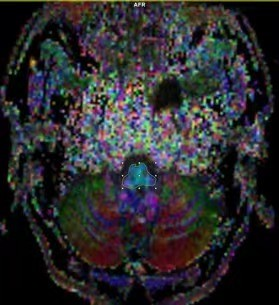
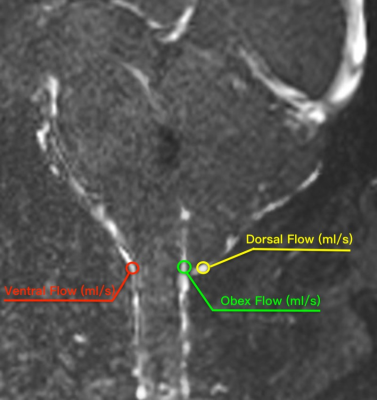
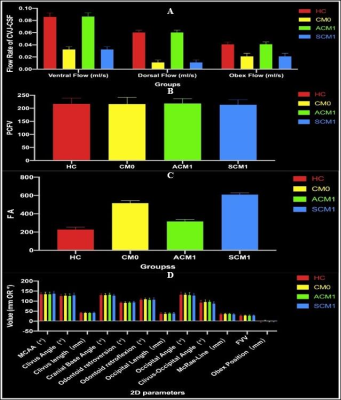
MR image:Between January 2021 and July 2022, 80 patients(25 males /55 females, age range: 33-58 years,mean:43.49±6.434 years,BMI range:17.7-32.8, mean: 23.34±3.0998)with CMI clinically diagnosed by MRI TIW and 20 healthy volunteers(5 males /15 females,age range:35-56 years,mean:40.49±3.434 years,BMI range:17.9-30.8,mean:21.89±2.61)were retrospectively selected for this study.All the examinations underwent at a 3T MRI scanner(MAGNETOM VIDA,Siemens Healthcare,Erlangen, Germany)and the details of parameters and are shown in Table 1.Reconstruction & Segmentation:2D morphometric variables of the craniocervical junction(CVJ), posterior cranial fossa(PCF)were measured on the sagittal T1-weighted MRI images(Figure 1).Volumes of PCF(PCFV)were measured on 3D-MPRAGE images by iPlan, BrainLab. The DTI and tractography data of CVJ were obtained by DTI procession software(Syngo.Via;Siemens Healthineers)(Figure 2).The hydrodynamics of CVJ-CSF were analyzed using PC-MRI(Figure 3).
Statistics:The parameters of morphology,neurobiology,and hydrodynamics from the 4 groups healthy control (HC),CM0, ACMI,SCMI,were statistically compared using the One-way ANOVA with a significant level set to be p<0.05.
Results
According to T1W images and symptomatic or not CMI,all the patient were divided into 3 groups,HC(n= 20)、CM0(n=20)、ACMI(n=20)、SCMI(n=20).2D morphometric variables of CVJ including MCAA (°), Clivus Angle (°), Clivus length(mm),Clivus length (mm),Cranial Base Angle (°),Odontoid retroversion(°), Odontoid retroflexion(°),Occipital Length (mm),Occipital Angle (°),Clivus-Occipital Angle(°), McRae-Line (mm), FVV-Line (mm), and Obex Position (mm), were not statistically significant(p>0.05),and 3D morphometric variables of volume of PCF(PCFV) were also not statistically significant p>0.05)(Figure 4).However, fractional anisotropy from DTI quantitative parameters show significantly difference in 4 groups (p=0.00). Cerebrospinal fluid(CSF)flow from ventral, dorsal and obex of CVJ had significant differences in all groups (all p<0.01).Discussion
Although radiology is an important tool in the diagnosis of CMI,but our conclusions and numerous previous studies suggest that CMI patients are associated with morphological malformations due to congenital dysplasia,there was no correlation between the severity of symptoms and 2D or 3D morphological parameters6-8.The measurements of morphological parameter were susceptible to demographic factors such as gender,age,race,BMI,and even imaging modality(CT/MRI)9-11.This was the reason why the 2D/3D morphological parameters measured in patients with CMI in different studies had significantly different results.The FA values from symptomatic CMI and CM0 were significantly increased,which suggested that microdamage for the neural structures of the CVJ in patients with CMI was not directly related to submicrocephalic tonsillar herniation.FA values were maker of axonal integrity,which is increased reflecting changes in white matter microstructure,possibly due to neural validation produced by headache in CMI.The FLOW was significantly lower in CM0 patients,and it was evident that their reduced FLOW was not directly related to the degree of cerebellar tonsillar herniation in craniocervical junction(CVJ),but might be closely related to the reduced pulsation of the cerebellar tonsils and the dysfunction of the myodural bridge complex.Conclusions
MR neurobiology and hydrodynamics were possible to distinguish well between different clinical and radiologic manifestations of CMI. FA values explain the relationship between white matter fiber tract inflammation and pain,while CSF flow velocity explains the relationship between cerebellar subungual tonsillar herniation and pain of CMI.In the future, the new MRI technology will play an even more important role in the diagnosis and treatment of CMI.Acknowledgements
No acknowledgement found.References
1.Ebrahimzadeh SA, Loth F, Ibrahimy A,et al. Diagnostic utility of parasagittal measurements of tonsillar herniation in Chiari I malformation. Neuroradiol J. 2022;35(2):233-239.
2. Antkowiak L, Rogalska M, Stogowski P, et al. Clinical Application of Diffusion Tensor Imaging in Chiari Malformation Type I- Advances and Perspectives. A Systematic Review. World Neurosurg. 2021;152:124-136.
3. Gok H, Naderi S.Prognostic Value of Craniovertebral Junction Diffusion Tensor Imaging in Patients with Chiari Type 1 Malformation. Turk Neurosurg. 2020;30(3):400-406.
4. Houston JR, Hughes ML, Bennett IJ, et al. Evidence of Neural Microstructure Abnormalities in Type I Chiari Malformation: Associations Among Fiber Tract Integrity, Pain, and Cognitive Dysfunction [published correction appears in Pain Med. 2021 May 05;:]. Pain Med. 2020;21(10):2323-2335.
5. Krishna V, Sammartino F, Yee P, et al. Diffusion tensor imaging assessment of microstructural brainstem integrity in Chiari malformation Type I. J Neurosurg. 2016;125(5):1112-1119.
6. Eppelheimer MS. Identification of Chiari Malformation Type I Brain Morphology and Biomechanics: A Multi-Faceted Approach to Determine Diagnostic and Treatment Criteria. University of Akron; 2020.
7. Khalsa SSS, Geh N, Martin BA, et al. Morphometric and volumetric comparison of 102 children with symptomatic and asymptomatic Chiari malformation Type I. J Neurosurg Pediatr. 2018;21(1):65-71.
8.Thakar S, Kanneganti V, Talla Nwotchouang BS, et al. Are Two-Dimensional Morphometric Measures Reflective of Disease Severity in Adult Chiari I Malformation?. World Neurosurg. 2022;157:e497-e505.
9. Iqbal S, Robert AP, Mathew D. Computed tomographic study of posterior cranial fossa, foramen magnum, and its surgical implications in Chiari malformations. Asian J Neurosurg. 2017;12(3):428-435.
10. Roller LA, Bruce BB, Saindane AM. Demographic confounders in volumetric MRI analysis: is the posterior fossa really small in the adult Chiari 1 malformation?. AJR Am J Roentgenol. 2015;204(4):835-841.
11. Hussain I, Winston GM, Goldberg J, et al. Impact of imaging modality, age, and gender on craniocervical junction angles in adults without structural pathology. J Craniovertebr Junction Spine. 2019;10(4):240-246.
Figures