1182
Age-related Vascular Changes in Choroid Plexus Evaluated Using High-resolution USPIO-Enhanced 7T MRI1Department of Radiology, New York University Grossman School of Medicine, New York, NY, United States, 2Vilcek Institute of Graduate Biomedical Sciences, New York University Grossman School of Medicine, New York, NY, United States
Synopsis
Keywords: Neurofluids, Aging
The choroid plexus (ChP) is a highly vascularized structure that is important in CSF production and waste solute clearance. In this work, USPIO-enhanced 7T imaging was used to detect microvascular changes associated with normal aging. We showed improved susceptibility contrast of ChP vessels and surrounding stromal tissues on high-resolution USPIO-enhanced 2D T2*-weighted MRI. Both age-related sparser vasculature and distended stromal tissues were identified. ChP volume increases with age, whereas the difference between pre-/post-contrast quantitative susceptibility map (QSM) decreases with age (P<0.05); and vascular degenerative change may occur earlier than volume change.INTRODUCTION
The choroid plexus (ChP) within the ventricles is part of the blood-cerebrospinal fluid (CSF) barrier and consists of a villous epithelial-endothelial vascular tissue mass for CSF production. It is organized as tight epithelium enclosing a highly vascularized stromal core that contains fenestrated capillaries and connective tissues [1]. An increasing number of studies have uncovered its potential role in waste solute clearance associated with cognitive impairment and Alzheimer's disease [2]. Previous MRI studies mainly focused on age-related volume changes of ChP using structural MRI [3]. Since ChP is a highly vascularized structure, in this study, we used ultra-high field MRI with the administration of ultrasmall super-paramagnetic iron oxide (USPIO) to detect age-related vascular changes and their association with stromal volume. USPIO-enhanced 7T MRI offers superior imaging resolution and susceptibility contrast, which is ideal for differentiating and characterizing vascular changes in ChP from surrounding connective tissue changes in the aging process.METHODS
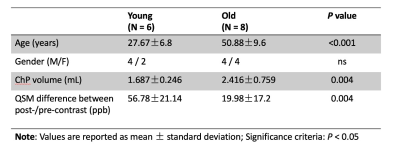
Fourteen healthy participants (age range: 21-70 years old, 40.83±14.46; 8 males/6 females) were scanned at 7T using a 32-channel head coil. The imaging protocol included: (1) T1-weighted MPRAGE (spatial resolution = 1mm3 isotropic) before USPIO (Ferumoxytol) contrast administration; (2) 2D gradient echo (GRE) sequence (TR/TE=1250/25ms, voxel size=0.25*0.25*2mm3) during contrast administration, and (3) adapted 3D susceptibility weighted imaging (SWI) (TR=22ms, TE1/TE2=7.5/15ms, voxel size = 0.25*0.25*1mm3) before and after contrast administration [4]. ChP was manually segmented based on the T1-weighted image. SWI images were generated by applying the unwrapped phase mask [5]. SHARP algorithm combined with the iSWIM method was used to produce quantitative susceptibility mapping (QSM) based on 3D SWI data [6, 7]. Pre-/post-contrast QSM subtraction map was calculated to inform the susceptibility alteration within vessels induced by the contrast agent. Participants were divided into young and old groups using an age cutoff of 40 years old. Independent t-tests were applied to reveal the group differences between young (≤40 years old, age=27.67±6.8, 4 males/2 females) and old (>40 years old, age=50.88±9.6, 4 males/4 females) subjects (Table 1). Pearson correlations were calculated to reveal the relationship between age and ChP volume and the pre-/post-contrast QSM difference.RESULTS
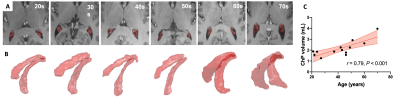
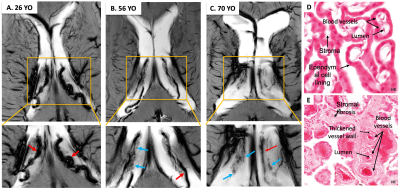
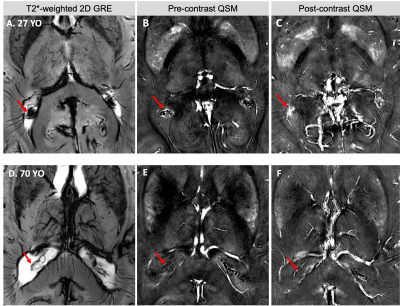
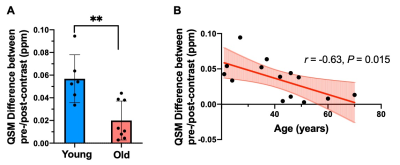
The 3D volume rendering results from 3D MPRAGE showed that ChP volume is increased with age and is more prominent towards the posterior horn (Fig. 1). Pearson's correlation result showed the volume was positively correlated with age (r = 0.79, P < 0.001). USPIO-enhanced high-resolution 2D GRE has unique T2*- and T2-weighted contrast with detailed delineation of ChP vasculature (dark signal) surrounded by stromal tissue (gray signal) within ventricle (bright signal) (Fig. 2). ChP of the older subject showed a thinner and sparser vasculature (red arrows) surrounded by enlarged cloudy-appearance stroma tissues (blue arrows) as compared to the younger subject (Fig. 2A-C). These findings are consistent with histopathology studies using hematoxylin-eosin (HE) staining (Fig. 2D-E), in which the ChP of an old subject manifested as a thickened vascular wall with a narrower lumen and distended fibrotic stroma [8]. In a young subject, a well-defined hypointense ChP in the posterior horn of ventricle showed greater enhancement on post-contrast QSM image (Fig. 3A-C) consistent with higher vasculature. Whereas, ChP in the old subject was enlarged but less enhanced or vascularized engaged by cystic alterations (red arrow) (Fig. 3D-F). The difference between Post- and pre-contrast QSM within ChP was significantly smaller in old than young subjects, and such QSM difference was negatively correlated with age (r = -0.63, P = 0.01) (Fig. 4).DISCUSSION
This initial work demonstrated that USPIO-enhanced 7T MRI using T2*-weighted 2D and 3D GRE can offer anatomical insights related to ChP vasculature changes during normal aging, which are invisible with conventional MRI. It has been reported from histological studies that vascular changes occur in people as early as in their 40s [9], therefore we choose 40 instead of 65 as the age cutoff to divide young and old to reflect early microvascular alterations within ChP. Compared with young subjects, old subjects showed vascular shrinkage and distended stromal tissues with fibrotic and cystic formation, consistent with findings in the histological studies. Changes in the ChP microvasculature may precede the volume change and has the potential to become an early imaging marker of ChP degeneration. These findings indicated microvascular aging, stromal fibrosis, and subsequent blood-CSF filtration might be the basis of the enlarged volume observed in the structural MRI.CONCLUSION
USPIO-enhanced 7T data were utilized to evaluate the age-related ChP vascular changes in healthy participants (20-70 years old). Gradually reduced ChP vasculature was found with normal aging accompanied by increased connective tissue volume and stromal fibrosis and cystic formation. These results may help to better elucidate the underlying mechanisms regarding the degenerative ChP changes in the elderly and their role in brain waste clearance and cognitive impairment.Acknowledgements
We acknowledge the funding sources for this study: RF1 NS110041, R01 NS 108491, and R13 AG067684 from National Institutes of Health (NIH).References
1. Hubert, V., et al., Clinical Imaging of Choroid Plexus in Health and in Brain Disorders: A Mini-Review. Frontiers in Molecular Neuroscience, 2019. 12.
2. Choi, J.D., et al., Choroid Plexus Volume and Permeability at Brain MRI within the Alzheimer Disease Clinical Spectrum. Radiology, 2022. 304(3): p. 635-645.
3. Alisch, J.S.R., et al., Characterization of Age-Related Differences in the Human Choroid Plexus Volume, Microstructural Integrity, and Blood Perfusion Using Multiparameter Magnetic Resonance Imaging. Frontiers in Aging Neuroscience, 2021. 13.
4. Chen, Y., et al., An interleaved sequence for simultaneous magnetic resonance angiography (MRA), susceptibility weighted imaging (SWI) and quantitative susceptibility mapping (QSM). Magn Reson Imaging, 2018. 47: p. 1-6.
5. Haacke, E.M., et al., Susceptibility-Weighted Imaging: Technical Aspects and Clinical Applications, Part 1. American Journal of Neuroradiology, 2009. 30(1): p. 19-30.
6. Schweser, F., et al., Quantitative imaging of intrinsic magnetic tissue properties using MRI signal phase: An approach to in vivo brain iron metabolism? NeuroImage, 2011. 54(4): p. 2789-2807.
7. Haacke, E.M., et al., Susceptibility mapping as a means to visualize veins and quantify oxygen saturation. J Magn Reson Imaging, 2010. 32(3): p. 663-76.
8. Prineas, J.W., J.D.E. Parratt, and P.D. Kirwan, Fibrosis of the Choroid Plexus Filtration Membrane. Journal of Neuropathology and Experimental Neurology, 2016. 75: p. 855 - 867.
9. Thore, C.R., et al., Morphometric analysis of arteriolar tortuosity in human cerebral white matter of preterm, young, and aged subjects. J Neuropathol Exp Neurol, 2007. 66(5): p. 337-45.
Figures