1180
Modified dual-contrast multi-shot 3D EPI for distortion-free and fast acquisition of simultaneous MR angiography and venography (DEPSAV)1State Key Laboratory of Brain and Cognitive Science, Institute of Biophysics, Chinese Academy of Sciences, Beijing, China, 2University of Chinese Academy of Sciences, Beijing, China, 3The Innovation Center of Excellence on Brain Science, Chinese Academy of Sciences, Beijing, China, 4Siemens Shenzhen Magnetic Resonance Ltd, Shenzhen, China, 5Institute of Artificial Intelligence, Hefei Comprehensive National Science Center, Hefei, China
Synopsis
Keywords: Data Acquisition, Blood
GRE-based vascular imaging suffers from the low acquisition efficiency. In this study, we presented a new 3D dual-contrast multishot-EPI based acquisition method called DESPAV for simultaneous MR angiography and venography. Full flow compensation for K-space center line and novel Center-out trajectory was implemented. A reconstruction pipeline was developed to correct for inter-shot phase errors, off-resonance induced artifact, and enable distortion-free multi-shot joint reconstruction. Preliminary results showed that DEPSAV can provide fast intracranial arterial and venous vasculature depiction with comparable contrast and image quality to 3D GRE-based TOF/SWI, while achieve ~3-fold reduction in acquisition time.Introduction
Time-of-flight (TOF) MR angiography (MRA) and susceptibility-weighted MR venography (MRV) are widely used in the examination of cerebral vessels. However, the mainstream acquisition of MRA and MRV is based on gradient-echo (GRE) sequence and thus is limited in the acquisition efficiency, even with its multi-echo variant 1. Recently, 3D multi-shot echo-planar imaging (ms-EPI) was introduced to acquire TOF or SWI images separately, with significantly reduced scanning time2,3. Nevertheless, EPI has the following drawbacks for vascular imaging: (i) the flow-induced phase variation causes variable “ghost” artifact associated with the K-space trajectory; (ii) the long effective TE (TEeff) reduces the TOF contrast and introduces a blurring effect; (iii) low bandwidth in phase-encoding direction causes geometric distortion arising from B0-inhomogeneity; (iv) the shot-to-shot phase variation caused by eddy current and physiological noise needs to be resolved in ms-EPI. To overcome these problems and combined the acquisition of TOF and SWI images, we proposed the flow-compensated multi-shot Dual-contrast 3D EPI for Simultaneous MRAV (DEPSAV). The method consists of flow compensation, the center-out trajectory, and postprocessing workflow including phase correction and distortion-free reconstruction for highly-segmented ms-EPI. The feasibility of DEPSAV for fast cerebral angiography and venography was evaluated.Method
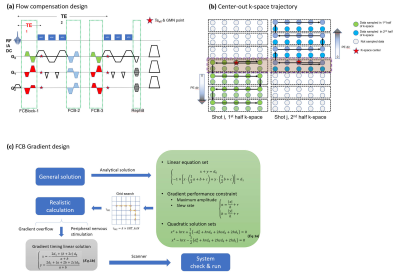
Theory: Sequence design- Flow compensation
- Center-out reordering
The echo-train length (ETL) was limited in DEPSAV since the background suppression for TOF-contrast was impaired at long TR. In this study, the ETL was set to 9 with TR=49ms to compromise between the arterial and venous contrasts. With Ny=272 and centric segment overlapped, the total shot number was 16×2=32, and the effective undersampling factor Reff≈30, which was a highly ill-posed inverse problem for reconstruction, let alone the capacity of parallel-imaging for further acceleration.
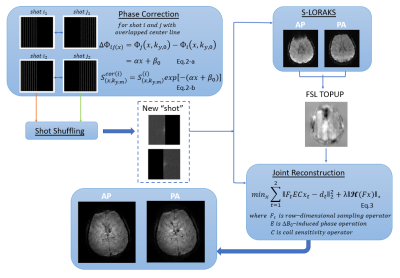
Theory: Reconstruction
- Shot combination
- Distortion-free reconstruction
Experiment
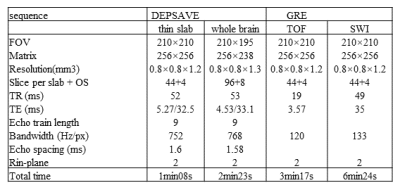
Simulation was performed to demonstrate the ghost artifact induced by off-resonance using center-out ordering. Standard resolution (0.8×0.8×1.2mm3) images of DEPSAV were obtained in phantom and in-vivo and compared with conventional GRE on a Siemens 3T Prisma system. The key parameters were shown in Table.1.
Results
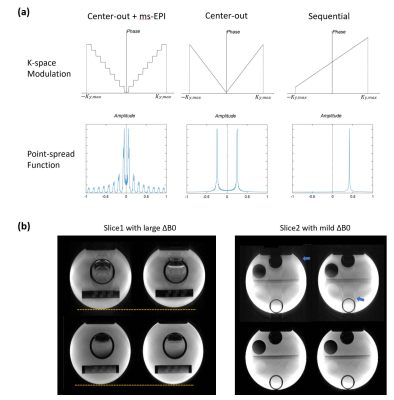
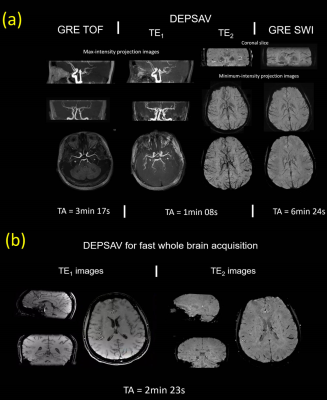
Fig3.(a) showed that the ghost kernel of point-spread-function (PSF) caused by the highly discontinuous modulation with acquisition scheme of DEPSAV, which was different from the ‘split’ artifact of single-shot center-out EPI or the displacement of sequential EPI10. Fig.3(b),(c) showed the effect of ghost elimination and distortion correction by the field-map informed reconstruction.Compared with GRE TOF-MRA, the TE1 images of DEPSAV provided angiography with acceptable contrast between flow and tissue, although the background suppression was suboptimal due to the long TR.
Compared with GRE SWI, the TE2 images of DESPAV achieved similar quality in depicting the cerebral veins. Moreover, the high-frequency k-space was filled later than TEeff in the echo train of center-out ordering, which improved the capability of DEPSAV to capture smaller vessels and subtle structures.
Discussion & Conclusion
We presented DEPSAV, a dual-contrast multi-shot 3D EPI for fast and simultaneous acquisition of cerebral angiography and venography. Preliminary results show that the whole-brain TOF and SWI contrast images with 0.8×0.8×1.2mm3 resolution can be collected within 200s. The highly discontinuous k-space phase modulation due to the center-out reordering and multi-shot acquisition was successfully corrected by shot-shuffling and BUDA-style reconstruction.Further improvements of DEPSAV such as multiple-overlapping thin-slabs techniques are still in progress. SVD-free optimization methods will improve the reconstruction speed. The higher isotropic-resolution acquisition will be evaluated on patients with cerebrovascular diseases to demonstrate its clinical values.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (82271985, 82001804, 8191101305), the Ministry of Science and Technology of China (2022ZD0211901, 2019YFA0707103), and the Natural Science Foundation of Beijing Municipality (7191003).References
1. Du YP, Jin Z. Simultaneous acquisition of MR angiography and venography (MRAV). Magn Reson Med. 2008;59(5):954-958.
2. Liu W, Zhou K. 3D Flow Compensated Interleaved EPI with Partial Fourier Acquisition: A Feasibility Study for Fast Intracranial TOF-MRA. Proc. Intl. Soc. Mag. Reson. Med. 28 (2020)0535
3. Liu W, Zhou K. 3D Flow Compensated Interleaved EPI with a Centric Reordering Scheme for Fast High-Resolution Susceptibility-Weighted. Proc. Intl. Soc. Mag. Reson. Med. 28 (2020)0535
4. Beck G, Li D, Haacke EM, Noll TG, Schad LR. Reducing oblique flow effects in interleaved EPI with a centric reordering technique. Magn Reson Med. 2001;45(4):623-629.
5. Liao C, Bilgic B, Tian Q, et al. Distortion-free, high-isotropic-resolution diffusion MRI with gSlider BUDA-EPI and multicoil dynamic B0 shimming. Magn Reson Med. 2021;86(2):791-803.
6. Hetzer S, Mildner T, Möller HE. A Modified EPI sequence for high-resolution imaging at ultra-short echo time: Modified EPI for Ultra-Short TE. Magn Reson Med. 2011;65(1):165-175.
7. Mani M, Jacob M, Kelley D, Magnotta V. Multi-shot sensitivity-encoded diffusion data recovery using structured low-rank matrix completion (MUSSELS): Annihilating Filter K-Space Formulation for Multi-Shot DWI Recovery. Magn Reson Med. 2017;78(2):494-507.
8. Haldar JP. Low-Rank Modeling of Local $k$-Space Neighborhoods (LORAKS) for Constrained MRI. IEEE Trans Med Imaging. 2014;33(3):668-681.
9. Andersson JLR, Skare S, Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. NeuroImage. 2003;20(2):870-888.
10. Bender JA, Ahmad R, Simonetti OP. The importance of k -space trajectory on off-resonance artifact in segmented echo-planar imaging. Concepts Magn Reson Part A. 2013;42A(2):23-31.
Figures