1177
Capillary-weighted velocity-selective ASL for subsecond fast fMRI1Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 2Department of Radiology, Harvard Medical School, Boston, MA, United States, 3Department of Biomedical Engineering, Boston University, Boston, MA, United States, 4Division of Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, MA, United States, 5Center for Functional Magnetic Resonance Imaging, Department of Radiology, University of California San Diego, La Jolla, CA, United States
Synopsis
Keywords: Data Acquisition, fMRI
This work evaluates capillary-weighted VS-ASL for subsecond functional cerebral blood flow (CBF) mapping with high microvascular specificity. Three experiments were conducted: one to demonstrate that capillary-weighted VS-ASL is mostly free from macrovascular contamination when compared to arterial-weighted VS-ASL, one to demonstrate that functional MRI is feasible with capillary-weighted VS-ASL at moderate repetition times, and one to show that, despite lower sensitivity, capillary-weighted functional MRI can be performed at subsecond temporal resolution. Our findings suggest that capillary-weighted VS-ASL may enable the study of fast functional CBF responses with high neuronal specificity.Introduction
Velocity-selective arterial spin labeling (VS-ASL)1,2,3,4,5 was developed to reduce artifacts in ASL caused by arterial transit time (ATT) heteronegeity. By labeling blood based on velocity, the bolus arrival time becomes very short, allowing near immediate delivery to the microvasculature. This unique feature of VS-ASL could enable high temporal resolution functional perfusion imaging6 of rapidly varying neuronal activity. VS-ASL can image either arterial- or capillary-weighted signals1. While arterial-weighted signals can provided increased sensitivity, capillary-weighted signals are expected to provide increased neuronal specificity. If capillary-weighted fMRI could be performed at fast scales, functional contrast with unprecedented temporal and spatial specificity would be possible. Due to the low sensitivity, however, fast imaging of capillary-weighted signals has not been demonstrated. This work evaluates capillary-weighted VS-ASL for subsecond functional CBF mapping with high microvascular specificity. We first confirm that capillary-weighted VS-ASL is mostly free from macrovascular contamination, and then conduct two functional MRI experiments with visual stimulation, one at a moderate TR=1200ms, and one with a short TR=500ms to show that capillary-weighted VS-ASL is capable of tracking changes in perfusion with subsecond temporal resolution.Methods
Three human subjects were scanned using VS-ASL on a 3T Siemens Prisma scanner using a 32ch head-coil. Informed consent was obtained prior to scanning. A VS-ASL sequence was used with Vcut=2.4cm/s and a 2D-EPI readout with resolution of 3.5x3.5x7mm3 (experiments 1,2) and 3x3x7mm3 (experiment 3), PF 6/8, rBW=2502Hz/px, flip=90o, TE=22ms, matrix=64x64. Three slices were positioned over the calcarine sulcus. BOLD and perfusion weighted time-series were generated by surround addition and subtraction of tag-control images. Toggling bipolar gradients after the readout excitation allowed switching between arterial-weighted (gradients off) and capillary-weighted (gradients on) VS-ASL. For functional experiments, data were analysed using FSL FEAT (smoothing=3mm, highpass=100s) after motion correction with AFNI.Experiment-1: Arterial- vs. capillary-weighted VS-ASL
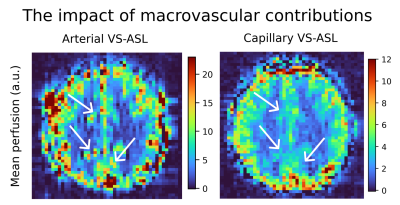
Arterial- and capillary-weighted images were acquired with $$$\tau$$$=700ms and TR=3000ms in one subject (39/M) to evaluate qualitative and quantitave tSNR (mean/std of timeseries) differences in perfusion maps from both modalities.
Experiment-2: Block-design capillary-weighted VS-ASL for fMRI
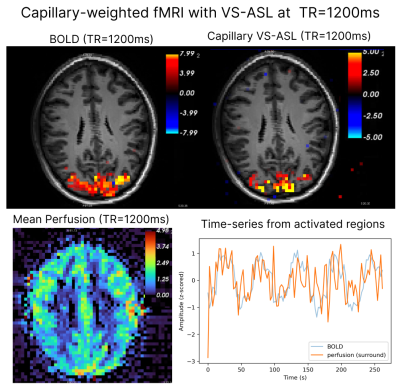
In a separate subject (23/M), images were acquired with $$$\tau$$$=750ms and TR=1200ms (four 4m30s runs averaged) during visual flickering checkerboard stimulation (25/30s ON/OFF blocks). A GLM was performed using the task design as regressor without additional confounds.
Experiment-3: Oscillatory-design subsecond capillary-weighted VS-ASL fMRI
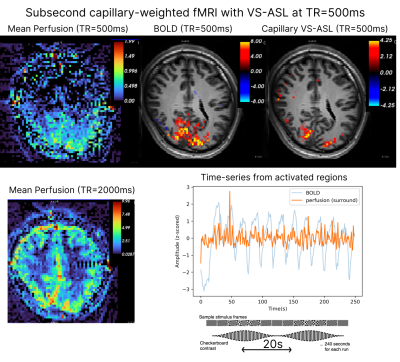
Capillary-weighted functional runs were acquired from a third participant (26/F) with $$$\tau$$$=340ms and TR=500ms (four 4m30s runs averaged) during stimulation with a visual flickering checkerboard with luminance contrast modulated at 0.05Hz. A GLM was performed against orthogonal oscillatory regressors with period of 20s. Activation was obtained as the Gaussianzed Z/F-statistics of the fit to both basis functions.
Results
Experiment-1Figure 1 illustrates perfusion maps from arterial- and capillary-weighted VS-ASL. Macrovascular components (manifesting as focal hyperintensities) seen in arterial-weighted VS-ASL are attenuated in capillary-weighted VS-ASL. tSNR was 2-4x larger for arterial-weighted when compared to capillary-weighted VS-ASL in the visual cortex.
Experiment-2
Figure 2 shows BOLD and capillary-weighted VS-ASL signals obtained with a TR=1200ms (effective perfusion sampling rate: 2400ms). Both BOLD and VS-ASL activation are clearly detectable, and the functional CNR is high. The mean perfusion map from the functional run shows gray-white differentiation, and the time-series from the active regions clearly follows the task paradigm.
Experiment-3
Figure 3 shows BOLD and capillary-weighted VS-ASL activation maps obtained with a temporal resolution of 500ms during an oscillating stimulus (effective perfusion sampling rate: 1000ms). Capillary-weighted changes could be detected despite lower tSNR. In the capillary-weighted activation maps, peak sites of activation localize to gray matter, in contrast to BOLD where highest activity concentrates next to draining veins.
Discussion
Our results suggest capillary-weighted VS-ASL is subtantially less contaminated by macrovasculature, though the tSNR is substantially lower than the arterial-weighted counterpart. For increasingly shorter $$$\tau$$$, the majority of the velocity-selective bolus may still remain within the pial arteries without macrovascular penetration. Lowering the velocity cutoff can mitigate this effect, but is limited by diffusion and eddy-current artifacts. For fMRI, our preliminary work on subsecond perfusion imaging indicates that capillary-weighted fMRI is possible at moderately short and subsecond $$$\tau$$$ /TR combinations and may result in greater specificity than BOLD. We find evidence for higher specificity by contrasting the location of activity in BOLD and perfusion maps, and noting that largest activation is not co-localized in both modalities, and perfusion is largest within gray matter. In our first functional experiment we used a moderate TR=1200ms to test the feasibility of fMRI with capillary-weighted VS-ASL. We note that TR=1200ms approaches the fastest perfusion imaging theoretically possible with conventional, spatial-based ASL modalities7, and is considerably less contaminated by macrovasculature. It was possible to find activity even in single-runs (not shown), and see task-locked activity qualitatively. Experiment 3 demonstrated feasibility of using capillary-weighted VS-ASL for ultrafast fMRI with a $$$\tau$$$ of 340ms and a TR of 500ms resulting in activity more localized to gray matter, again suggesting higher specificity despite a high sensitivity cost. Next steps to improve sensitivity include optimizing slab-selective VS-ASL8,9, shortening the TE, improving labelling efficiency of the VS module, and potentially using VS inversion10 instead of VS saturation.Conclusion
Subsecond fMRI is feasible with capillary-weighted VS-ASL, which may enable the study of fast functional responses with high neuronal specificity11,12.Acknowledgements
This work was funded by NIH grants P41-EB015896, R21-NS106706, R01-EB019437, P41-EB030006, R00-MH111748, R01-AG070135, S10-OD023637 and S10-RR019371.References
[1] Eric C. Wong, Thomas T. Liu, Karam Sidaros, Lawrence R. Frank, and Richard B. Buxton. Velocity selective arterial spin labeling. In Proceedings of the 10th Annual Meeting of ISMRM, Honolulu, HI, USA, page 621, 2002.
[2] Eric C. Wong, Matthew Cronin, Wen-Chau Wu, Ben Inglis, Lawrence R. Frank, and Thomas T. Liu. Velocity-selective arterial spin labeling. Magnetic Resonance in Medicine, 55(6):1334–1341, 2006.
[3] David G. Norris and Christian Schwarzbauer. Velocity selective radiofrequency pulse trains. Journal of Magnetic Resonance, 137(1):231–236, 1999.
[4] Guillaume Duhamel, Cédric de Bazelaire, and David C. Alsop. Evaluation of systematic quantification errors in velocity-selective arterial spin7labeling of the brain. Magnetic Resonance in Medicine, 50(1):145–153, 2003.
[5] Qin Qin, David C. Alsop, Divya S. Bolar, Luis Hernandez-Garcia, James Meakin, Dapeng Liu, Krishna S. Nayak, Sophie Schmid, Matthias J. P. van Osch, Eric C. Wong, Joseph G. Woods, Greg Zaharchuk, Moss Y. Zhao, Zungho Zun, Jia Guo, and the ISMRM Perfusion Study Group. Velocity-selective arterial spin labeling perfusion MRI: a review of the state of the art and recommendations for clinical implementation. Magnetic Resonance in Medicine, 88(4):1528–1547, 2022.
[6] Luis Hernandez-Garcia, Jon-Fredrik Nielsen, and Douglas C. Noll. Improved sensitivity and temporal resolution in perfusion fMRI using velocity selective inversion ASL. Magnetic Resonance in Medicine,81(2):1004–1015, 2018.
[7] Eric C Wong, Wen-Ming Luh, and Thomas T Liu. Turbo ASL: arterial spin labeling with higher SNR and temporal resolution. Magnetic Resonance in Medicine, 44(4):511–515, 2000.
[8] Divya S. Bolar, Jonathan R. Polimeni, Ned Ohringer, Elfar Adalsteinsson, and Bruce R. Rosen. Turbo VSASL: slice-and velocity-selective ASL for high temporal resolution functional CBF mapping. In Proceed- ings of the 26th Annual Meeting of ISMRM, Paris, France, page 710, 2018.
[9] Joseph G. Woods, Eric C. Wong, Emma C. Boyd, and Divya S. Bolar. Vespa ASL: Velocity and spatially selective arterial spin labeling. Magnetic Resonance in Medicine, 87(6):2667–2684, 2022.
[10] Qin Qin and Peter C.M. van Zijl. Velocity-selective-inversion prepared arterial spin labeling. Magnetic Resonance in Medicine, 76(4):1136– 1148, 2015.
[11] Laura D. Lewis, Kawin Setsompop, Bruce R. Rosen, and Jonathan R. Polimeni. Fast fMRI can detect oscillatory neural activity in humans. Proceedings of the National Academy of Sciences, 113(43):E6679–E6685, 2016.
[12] Jonathan R. Polimeni and Laura D. Lewis. Imaging faster neural dynamics with fast fMRI: a need for updated models of the hemodynamic response. Progress in Neurobiology, 207:102174, 2021.
Figures