1167
AI-Assisted Iterative Reconstruction for CMR Multitasking1Biomedical Imaging Research Institute, Cedars-Sinai Medical Center, Los Angeles, CA, United States, 2Department of Bioengineering, University of California, Los Angeles, Los Angeles, CA, United States
Synopsis
Keywords: Image Reconstruction, Machine Learning/Artificial Intelligence
CMR Multitasking is a promising approach for quantitative imaging without breath-holds or ECG monitoring but standard iterative reconstruction is too long for clinical use. Supervised artificial intelligence (AI) can accelerate reconstruction but lacks generalizability and transparency, and T1 mapping precision has not been sufficient. Here we propose an AI-Assisted Iterative (AAI) reconstruction which takes an AI reconstruction output as a “warm start” to a well-characterized iterative reconstruction algorithm with only 2 iterations. The proposed method produces better image fidelity and more precise T1 maps than other accelerated reconstruction methods, in less than 15 seconds (16x faster than conventional iterative reconstruction).
Introduction
CMR Multitasking is a promising approach for quantitative imaging without breath-holds or ECG monitoring1, but slow iterative non-Cartesian reconstruction is a barrier to clinical adoption. Clinically feasible reconstruction time has been demonstrated using supervised artificial intelligence (AI)2,3, but as with any supervised AI, generalizability and transparency are open questions, especially in a clinical setting4. The T1 mapping precision of AI reconstruction also has room to improve. To retain the goal of fast, accurate image reconstruction without sacrificing generalizability or transparency, we propose an AI-Assisted Iterative (AAI) reconstruction method for CMR multitasking. The proposed method takes an AI reconstruction output as a “warm start” to a well-characterized iterative reconstruction algorithm with only 2 iterations, with the goal of producing better image fidelity and T1 mapping quality than either pure AI or pure 2-iteration reconstruction.Methods
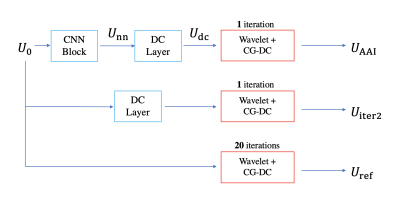
Reconstruction methods: To assess the performance of AI-assisted iterative reconstruction, we compare three different reconstruction methods (Figure 1) for determining the spatial factor U in T1 CMR multitasking5:a) Pure AI reconstruction Udc using a Convolutional Neural Network (CNN) block and a data-consistency (DC) layer3 with a zero-filled input U0
b) Pure iterative wavelet reconstruction Uiter2 from the initial guess U0 (2 iterations). The 2 iterations are one inverse Toeplitz DC layer3 (to match [a] above) and one alternating direction method of multipliers (ADMM)-based wavelet-thresholding + conjugate-gradient (CG)-DC iteration
c) The proposed AI-assisted iterative reconstruction UAAI, which is obtained from one CNN block, one DC layer, and one ADMM wavelet-thresholding + CG-DC iteration.
All three methods are compared to the reference reconstruction Uref that uses 20 ADMM wavelet-thresholding + CG-DC iterations, initialized from U0.
Dataset overview: A total of 120 T1 CMR Multitasking datasets were collected from three Siemens 3T scanners. We used 96 cases for training the CNN network, 12 cases for validation and 12 cases for testing. The FOV for acquisition is 540 x 540 mm2 and the spatial resolution is 1.7 x 1.7 mm2. The matrix size of spatial factors is 320×320×32.
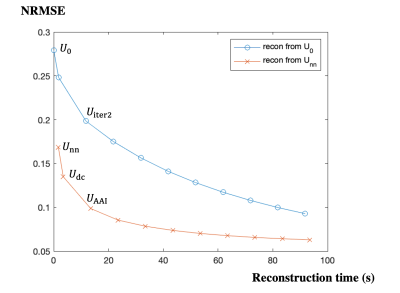
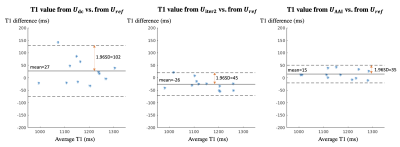
Evaluation methods: The spatial factors reconstructed by the three methods (Udc, Uiter2 and UAAI) were compared against the reference Uref using Normalized Root Mean Square Error (NRMSE) for the testing set. To better visualize the improvement of AAI reconstruction compared to pure iterative reconstruction, we extended each method to up to 9 ADMM iterations of wavelet-thresholding + CG-DC, and plotted the relationship between reconstruction time and NRMSE of each method. For the testing set, T1 maps were fitted from Udc, Uiter2, UAAI and Uref. Bland-Altman plots of the myocardial septal T1 values from Udc, Uiter2, UAAI vs. from Uref were calculated to evaluate the T1 mapping accuracy and precision for each method.
Results
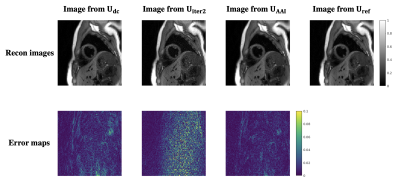
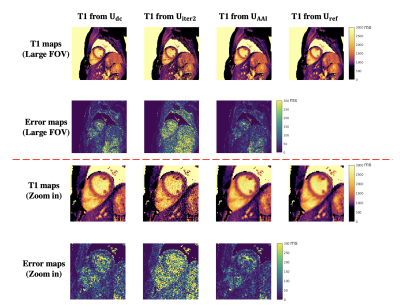
The reconstruction time of the reference Uref was 220 s. Figure 2 shows the relationship between reconstruction time and spatial factor NRMSE of pure iterative reconstruction (from U0) and the proposed AAI reconstruction (from Unn). The AAI curve is consistently lower than pure iterative reconstruction curve, which suggests the proposed method has better reconstruction fidelity when using similar reconstruction time. With the reconstruction time of 13.5 s, the proposed UAAI has similar NRMSE to 82-second pure iterative reconstruction. Figure 3 shows the reconstructed images of one testing data with different methods as well as their corresponding error maps. The proposed UAAI method has the least error among all methods. Figure 4 shows the T1 maps of the same testing data as Figure 3, where the proposed method has the best T1 map quality and the least T1 error. Figure 5 shows the Bland-Altman plots of the myocardial septal T1 values from Udc, Uiter2, UAAI vs. from Uref. The limits of agreements of T1 values from Udc, Uiter2, UAAI are ±102 ms, ±45 ms and ±35 ms, respectively.Discussion
Compared to the reference method Uref, the proposed UAAI accelerates reconstruction by 16x and still produces high fidelity reconstructed images and precise T1 maps. Compared to the two-iteration reconstruction Uiter2, the proposed UAAI produces visually better reconstructed images and more precise T1 maps (improves the limits of agreements of septal T1 values by 22%) with similar reconstruction time. Compared to the pure AI reconstruction Udc, the proposed UAAI produces more precise T1 maps (improves the limits of agreements of septal T1 values by 66%), with only 10-second longer reconstruction time.Conclusion
We proposed an AI-Assisted iterative reconstruction method for CMR multitasking to accelerate CMR multitasking reconstruction and improve the quality and robustness the reconstruction. The proposed method produced better image fidelity and more precise T1 maps than other accelerated reconstruction methods, in less than 15 seconds.Acknowledgements
This work was partially supported by NIH R01 EB028146.References
1. Christodoulou AG, Shaw JL, Nguyen C, Yang Q, Xie Y, Wang N, Li D. Magnetic resonance multitasking for motion-resolved quantitative cardiovascular imaging. Nature Biomed Eng 2018;2(4):215-226.
2. Chen Y, Shaw JL, Xie Y, Li D, Christodoulou AG. Deep learning within a priori temporal feature spaces for large-scale dynamic MR image reconstruction: Application to 5-D cardiac MR Multitasking. Med Image Comput Comput Assist Interv 2019:495-504.
3. Chen Z, Chen Y, Xie Y, Li D, Christodoulou AG. Data-Consistent Non-Cartesian Deep Subspace Learning for Efficient Dynamic MR Image Reconstruction. 2022 IEEE 19th International Symposium on Biomedical Imaging (ISBI)2022. p 1-5.
4. Knoll F, Hammernik K, Kobler E, Pock T, Recht MP, Sodickson DK. Assessment of the generalization of learned image reconstruction and the potential for transfer learning. Magnetic Resonance in Medicine 2019;81(1):116-128.
5. Shaw JL, Yang Q, Zhou Z, Deng Z, Nguyen C, Li D, Christodoulou AG. Free-breathing, non-ECG, continuous myocardial T1 mapping with cardiovascular magnetic resonance multitasking. Magn Reson Med 2019;81(4):2450-2463.
Figures