1161
A Simultaneous Measurement of Opposite-Phase-Encoding EPI in a Single fMRI session for the Reduction of Acquisition Time and SAR at 7T
Seong Dae Yun1, Erhan Genc2, and N. Jon Shah1,3,4,5
1Institute of Neuroscience and Medicine 4, INM-4, Forschungszentrum Juelich, Juelich, Germany, 2Department of Psychology and Neurosciences, Leibniz Research Centre for Working Environment and Human Factors (IfADo), Dortmund, Germany, 3Institute of Neuroscience and Medicine 11, INM-11, JARA, Forschungszentrum Juelich, Juelich, Germany, 4JARA - BRAIN - Translational Medicine, Aachen, Germany, 5Department of Neurology, RWTH Aachen University, Aachen, Germany
1Institute of Neuroscience and Medicine 4, INM-4, Forschungszentrum Juelich, Juelich, Germany, 2Department of Psychology and Neurosciences, Leibniz Research Centre for Working Environment and Human Factors (IfADo), Dortmund, Germany, 3Institute of Neuroscience and Medicine 11, INM-11, JARA, Forschungszentrum Juelich, Juelich, Germany, 4JARA - BRAIN - Translational Medicine, Aachen, Germany, 5Department of Neurology, RWTH Aachen University, Aachen, Germany
Synopsis
Keywords: Pulse Sequence Design, fMRI, 7T, improved fMRI mapping accuracy, EPI, geometric-distortion correction, opposite phase-encoding and reduced acquisition time
In high-resolution fMRI using EPI, geometric distortions typically seen in reconstructed images significantly hinder the accurate mapping of activated voxels. One method to correct for distortions is to acquire EPI data with an opposite phase-encoding direction. However, this method is usually implemented with an additional run of the same protocol, leading to a redundant measurement of parallel imaging calibration scans. Here, we present an EPI scheme that measures the opposite-direction data in a single fMRI session, substantially reducing the total acquisition time. We demonstrate more accurate functional mapping with the distortion correction in submillimetre whole-brain visual fMRI at 7T.Introduction
The detection of fMRI signals requires high spatiotemporal imaging to effectively capture the dynamic haemodynamic responses and specify their activation site. As this imaging condition is well satisfied with EPI, the method has been widely used in numerous fMRI studies. The performance of EPI has now been far improved by virtue of the development of hardware and imaging techniques, which allows an fMRI acquisition with a submillimetre voxel size at ultra-high fields.1,2,3 However, the geometric deformation of tissue structures typically seen in the EPI images significantly hinders accurate mapping of activated voxels in high-resolution fMRI. One widely used method to correct the distortion is the acquisition of EPI data with an opposite phase-encoding direction.4,5,6 However, this method is usually implemented by applying an additional run of the same EPI protocol only with the change of the phase-encoding direction, which can lead to a redundant measurement of parallel imaging (PI) calibration scan. Therefore, this work aims to present a novel EPI scheme that additionally measures EPI data with the opposite phase-encoding direction in a single fMRI session. The method substantially reduces total acquisition time, particularly for fMRI with a large imaging matrix size for high spatial-resolution imaging and whole-brain coverage.Methods
Figure 1a depicts a schematic diagram of a typical fMRI run using the standard EPI scheme. Before the start of the actual fMRI scans, two preceding measurements, i.e. calibration and dummy scans, are performed, which are necessary for the reconstruction of acceleration techniques (e.g. PI)7,8 and for reaching a steady-state. Here, the PI calibration scans can be acquired with EPI. However, at ultra-high fields, they are usually acquired using a more field-inhomogeneity tolerant sequence, such as a conventional gradient-echo, to improve the reconstruction quality, as is the case of the present work. In the standard scheme, EPI with an opposite phase-encoding is performed by applying an additional run of the same protocol with a change in the encoding direction (Fig. 1b). However, this strategy still requires another complete acquisition of the PI calibration scan which makes the acquisition time and specific absorption rate (SAR) redundant. This issue can become more severe when the PI calibration scan is acquired with a gradient-echo sequence. To avoid this conflict, the proposed scheme adjusts the EPI acquisition such that the first two EPI scans in the dummy stage are acquired with the opposite phase-encoding direction (Fig. 1c). The feasibility of using the proposed scheme for fMRI was verified with whole-brain, submillimetre fMRI (0.73 × 0.73 mm2, 117 slices), designed with a visual checkerboard paradigm; the detailed imaging configuration is described in Fig. 2a. Data sets from a healthy volunteer screened with a standard safety procedure were acquired at a Siemens Magnetom Terra 7T scanner with a 1-Tx/32-Rx head coil.Results
Figure 2b shows reconstructed original- and opposite-phase-encoding images from the proposed method. The different distortion characteristics of the two images were effectively computed in ANTs (https://github.com/ANTsX/ANTs)9, and a distortion-corrected image was generated, depicting no significant visible loss of spatial resolution. Figure 3 shows the distortion-corrected results for the entire slices, demonstrating reliable correction performance for all slice locations. The distortion-corrected EPI images were co-registered to the anatomical scan (MP2RAGE), and its co-registration accuracy was assessed in comparison to that of the original, uncorrected EPI images. Figure 4 shows the results presented in three different sectional views. The MP2RAGE and EPI images are displayed alongside, each depicting the counterpart of the brain; the background noise of the unified MP2RAGE scan was cleaned by the script.10 It can be seen that the degree of matching between the MP2RAGE and EPI images is significantly enhanced in the distortion-corrected case when compared to the non-corrected one. This is observed particularly around the regions marked by yellow circles (i.e. ventricles) or red arrows. For the original and distortion-corrected fMRI data, the first-level analysis was individually performed based on a GLM model using SPM12 (https://www.fil.ion.ucl.ac.uk/spm/software/spm12/). Figure 5 shows activated voxels obtained with an uncorrected p-value < 0.001, which are overlaid on the co-registered MP2RAGE scan. The results at the representative slice locations show that the activated voxels from distortion-corrected EPI are more precisely localised along the cortical ribbon when compared to the original, uncorrected EPI.Discussion and conclusions
This work demonstrates the use of the proposed EPI scheme for fMRI, which acquires EPI data with the opposite phase-encoding direction in a single fMRI session. This strategy can effectively spare another acquisition time for the PI calibration scans, which was 95.94 seconds for the submillimetre-protocol tested here (0.73 × 0.73 mm2, 117 slices), making the method particularly applicable for time-critical clinical applications. The spared time can become even greater for an fMRI application employing a higher spatial resolution and a larger number of slices. The presented work also successfully verifies the EPI distortion correction using the data obtained from the proposed scheme. Consequently, more accurate functional mapping onto the co-registered anatomical scan was achieved. Moreover, the proposed scheme can also open a possibility of a more direct distortion correction of fMRI data via the online reconstruction platform (e.g. ICE- Gadgetron11), enabling its straightforward use in clinical diagnosis.Acknowledgements
No acknowledgement found.References
- Kay K, Jamison KW, Vizioli L, Zhang R, Margalit E, Ugurbil K. A critical assessment of data quality and venous effects in sub-millimeter fMRI. Neuroimage. 2019 Apr 1;189:847-869. doi: 10.1016/j.neuroimage.2019.02.006. Epub 2019 Feb 5. PMID: 30731246; PMCID: PMC7737092.
- Sharoh D, van Mourik T, Bains LJ, Segaert K, Weber K, Hagoort P, Norris DG. Laminar specific fMRI reveals directed interactions in distributed networks during language processing. Proc Natl Acad Sci U S A. 2019 Oct 15;116(42):21185-21190. doi: 10.1073/pnas.1907858116. Epub 2019 Sep 30. PMID: 31570628; PMCID: PMC6800353.
- Yun SD, Pais-Roldán P, Palomero-Gallagher N, Shah NJ. Mapping of whole-cerebrum resting-state networks using ultra-high resolution acquisition protocols. Human Brain Mapping. 2022;43(11):3386-3403. https://doi.org/10.1002/hbm.25855.
- de Hollander G, van der Zwaag W, Qian C, Zhang P, Knapen T. Ultra-high field fMRI reveals origins of feedforward and feedback activity within laminae of human ocular dominance columns. Neuroimage. 2021 Mar;228:117683. doi: 10.1016/j.neuroimage.2020.117683. Epub 2020 Dec 30. PMID: 33385565.
- Navarro KT, Sanchez MJ, Engel SA, Olman CA, Weldon KB. Depth-dependent functional MRI responses to chromatic and achromatic stimuli throughout V1 and V2. Neuroimage. 2021 Feb 1;226:117520. doi: 10.1016/j.neuroimage.2020.117520. Epub 2020 Nov 1. PMID: 33137474; PMCID: PMC7958868.
- Marquardt I, De Weerd P, Schneider M, Gulban OF, Ivanov D, Wang Y, Uludağ K. Feedback contribution to surface motion perception in the human early visual cortex. Elife. 2020 Jun 4;9:e50933. doi: 10.7554/eLife.50933. PMID: 32496189; PMCID: PMC7314553.
- Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. SENSE: sensitivity encoding for fast MRI. Magn Reson Med. 1999 Nov;42(5):952-62. PMID: 10542355.
- Griswold MA, Jakob PM, Heidemann RM, Nittka M, Jellus V, Wang J, Kiefer B, Haase A. Generalized autocalibrating partially parallel acquisitions (GRAPPA). Magn Reson Med. 2002 Jun;47(6):1202-10. doi: 10.1002/mrm.10171. PMID: 12111967.
- Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage. 2011 Feb 1;54(3):2033-44. doi: 10.1016/j.neuroimage.2010.09.025. Epub 2010 Sep 17. PMID: 20851191; PMCID: PMC3065962.
- https://github.com/JosePMarques/MP2RAGE-related-scripts
- https://github.com/gadgetron/gadgetron
Figures
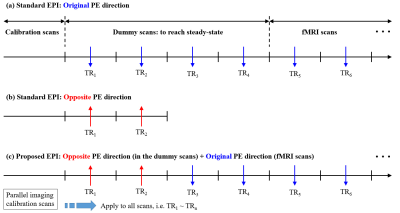
Figure 1. Schematic diagram of an fMRI
acquisition sequence using (a) standard
EPI with the original phase-encoding direction, (b) standard EPI with the opposite phase-encoding direction and (c) proposed EPI. The blue and red
arrows indicate the original and opposite directions, respectively. In this
work, the number of dummy and opposite EPI scans were set to 4 and 2, as shown
in the figure. The proposed EPI scheme acquires the parallel imaging
calibration scans only once for the reconstruction of both original- and
opposite-direction data, which is not the case for standard EPI.
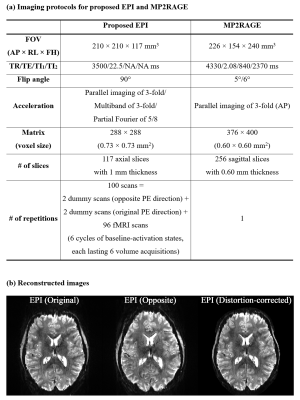
Figure 2. (a) Imaging parameters of submillimetre EPI and MP2RAGE scans used
in the visual fMRI experiment. (b) Reconstructed
images from the proposed method where each image panel (from left to right)
depicts results obtained from the original phase-encoding direction, opposite
phase-encoding direction and distortion correction using the ANTs processing.
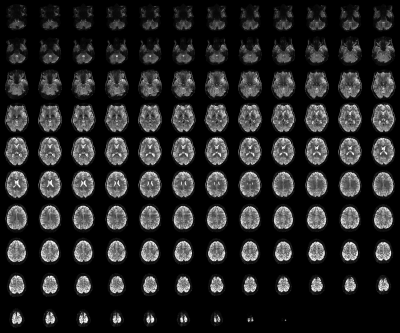
Figure 3. A distortion-corrected fMRI scan
from the time-series data (0.73 × 0.73
mm2, 117 slices). The corrected results did not show any significantly
visible loss of spatial resolution for any slice locations, demonstrating
reliable distortion correction performance.
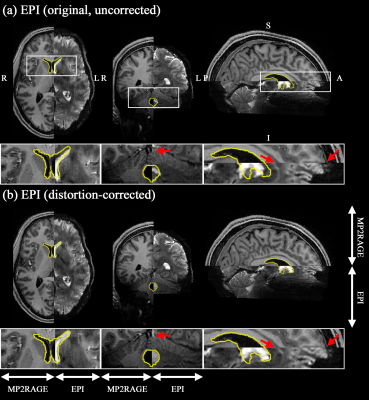
Figure 4. Results of co-registration to the
anatomical scan (MP2RAGE) from the (a)
original, uncorrected EPI and (b)
distortion-corrected EPI. For comparison purposes, The EPI and MP2RAGE scans
are displayed alongside, each depicting the counterpart of the brain. The
results from distortion-corrected EPI show improved co-registration accuracy,
particularly around the regions marked by yellow circles (i.e. ventricles) and
red arrows (see the enlarged depiction of the selected ROIs shown below in each
slice).
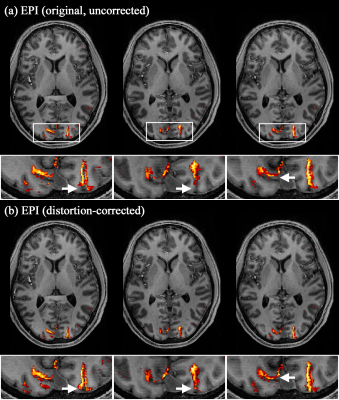
Figure 5. Functional analysis results obtained from the (a) original, uncorrected EPI and (b) distortion-corrected EPI data. The activated voxels were identified with an uncorrected p-value < 0.001 and are overlaid onto the co-registered MP2RAGE scan. The activated voxels from the distortion-corrected EPI data are more precisely localised along the cortical ribbon (see the white arrows in the enlarged depiction of the selected ROIs).
DOI: https://doi.org/10.58530/2023/1161