1149
Low spatial-frequency ripple artifacts in layer-fMRI EPI: Identification, cause, and mitigation strategies with Dual-polarity readout1Maastricht University, Maastricht, Netherlands, 2German Center for Neurodegenerative Diseases (DZNE), Bonn, Germany, 3Helen Wills Neuroscience Institute, University of California, Berkeley, CA, United States, 4Advanced MRI Technologies, Sebastopol, CA, United States, 5Siemens Medical Solutions USA, Inc., Berkeley, CA, United States, 6Brain Innovation, Maastricht, Netherlands, 7Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 8Graduate School of Informatics and Engineering, The University of Electro-Communications, Tokyo, Japan, 9National institutes of HEalth, Bethesda, MD, United States
Synopsis
Keywords: Artifacts, fMRI, layer-fMRI, UHF, EPI
High-resolution layer-fMRI has great potential to inform network-neuroscience. However, it is limited by EPI artifacts. Here, we discuss a class of fuzzy EPI ghosts arising from asymmetric trapezoidal gradients with ramp sampling. A meta analysis across layer-fMRI datasets finds this artifact everywhere, without exceptions. We believe that this artifact is constraining spatiotemporal resolutions more than SNR. In this abstract we aim to raise awareness for this artifact and evaluate mitigation strategies: dual-polarity EPI. We show that dual-polarity EPI allows layer-fMRI to break the barriers of current resolution limits: It allows 0.53mm imaging at 3T, and whole-brain 0.6mm fMRI at 7T.Purpose
High-resolution layer-fMRI has the potential to inform network neuroscience about causal neural information flow between brain areas. However, data collection is limited by EPI artifacts arising from inconsistencies between odd and even echoes. While conventional Nyquist ghosting is mitigated in common two-parameter phase correction (Ahn 1987; Heid 200, Feiweier 2013), layer-fMRI also suffers from additional higher-order effects:- Imperfections of gradient waveforms may increase with higher amplitude and slew rate and imperfections using large ramp sampling factors and low bandwidths of layer-fMRI protocols.
- Parallel imaging (vital in efficient layer-fMRI) relies on “known” aliasing. Thus, even small residual phase correction errors are amplified with GRAPPA/SENSE.
In this abstract, we aim to discuss a specific EPI artifact that arises from asymmetric trapezoidal gradient pulses at rising corners. When ramp sampling, this artifact manifests as blurry phase interference patterns at low spatial frequencies, often referred to as ‘low-frequency (fuzzy) ripples’. Here, we aim to characterize this artifact and understand how limiting it is in the research field of layer-fMRI (Fig. 1). Then, we seek to implement and test a potential mitigation strategy: complex-valued averaging of dual-polarity EPI readouts. We want to empirically test whether this mitigation strategy allows neuroimagers to overcome current resolution limits of conventional layer-fMRI protocols.
Methods
Experimental setupThree SIEMENS (Siemens Healthcare, Erlangen, Germany) scanners were used: 3T MAGNETOM PRISMA, classic MAGNETOM 7T, and the Feinbergatron MAGNETOM Terra Impulse Edition NextGen 7T (Feinberg 2021) with advanced gradient and RF hardware (Davids 2021, Gunamony 2022). We used a 3D-EPI sequence (Stirnberg 2021, Poser 2010), with optional alternating readout polarity.
Experiments assessing fuzzy ripples for overly pushy layer-fMRI protocols
We started with two layer-fMRI protocols that were previously established for robust layer-fMRI. A series of experiments was conducted with gradually increasing temporal and spatial resolutions, respectively (Fig. 2).
- A 0.8mm whole-brain protocol for 7T (Koiso 2022) was pushed to increase temporal resolution from 5.2s to 2.2s, by increasing GRAPPA factors.
- A 0.8mm slab protocol for 3T (Huber 2022) was pushed towards higher spatial resolutions up to 0.53mm.
Experiments assessing the efficacy of complex-values averaging with dual-polarity EPI
A combination of dual-polarity readouts to separate opposite polarity echoes with complex-valued averaging of their respective images has previously been proposed to mitigate off-resonance-induced ‘edge-ghosts’ (high spatial-frequency ghosts) in EPI-based fMRI acquisitions (van der Zwaag 2009, Poser 2013, Stirnberg 2022, Hu 1996 etc.). The working principle of this approach is schematically depicted in Fig. 3E-G. Reversed readout polarities are expected to completely invert the k-space shift pattern. Thus, the resulting EPI ghosts are expected to have the opposite phase for each readout polarity, and thus can be fully canceled with a complex-valued averaging. Here, we investigated whether this strategy can also address the low-frequency ripple artifacts.
We tested this approach with purposefully higher acceleration factors/resolutions than in conventional neuroscience application.
Data processing
Complex-valued averaging was performed after parallel image reconstruction and coil combination of IcePat (Jellúš 2014). Motion correction of complex-valued image space data with ANTS (Avants 2014). Layerification performed in LayNii (Huber 2021).
Results
We evaluated the list of 195 (human) layer-fMRI papers (https://layerfmri.com/papers) and performed a meta analysis across the subsection of studies that made their data publicly available (N=25). This meta analysis finds the fuzzy ripple artifact is visible across all datasets, without exceptions.Fig. 2 depicts how image quality degrades with more pushy protocols. With increasing spatiotemporal resolutions and GRAPPA factors, image artifacts become dominating. More so than salt-pepper noise. Fig. 4 and 5 show how complex-valued averaging can mitigate them efficiently.
Discussion
Based on the meta analysis (Fig. 1) and the experiment of gradually increasing spatiotemporal resolution (Fig. 2), we concluded that the fuzzy ripple artifact is not just an annoyance in conventional acquisition protocols (0.8mm 2-4a TRs). On the contrary, we find the results consistent with the notion that the fuzzy ripple artifact is the most limiting factor in current layer-fMRI acquisition protocols over SNR limits.The strategy of complex-valued averaging with dual-polarity EPI can have the disadvantage of temporal leakage, while keeping the update rate unchanged. This is comparable to slice-time-correction, effectively applying a temporal interpolation between neighboring TRs. Alternative reference-based dual-polarity EPI with complex-valued averaging (Fig 3E) can circumvent this limit at the cost of efficacy to account for dynamic phase errors (Van der Zwaag 2009, Hu 1996). Parallel imaging-based ghost mitigation methods (Hoge 2016)) that assume similar phase errors across the entire k-space have also been proposed to mitigate some forms of EPI ghosts and future empirical comparison studies are needed.
Summary
In this abstract, we raise awareness of a higher-order fuzzy ripple artifact, not previously discussed in the layer-fMRI literature, yet imposing significant constraints in modern layer-fMRI protocols. We find that dual-polarity EPI can mitigate this artifact effectively, overcoming previous resolution limits.Acknowledgements
- We thank all members of the layer-fMRI community that made their data publicly available. This provided the material for the meta-analysis in Fig. 1.
- Data shown in Fig. 2, 4A, and 5 were acquired with the kind support of scannexus.
- Gradient trajectories in Fig. 3A were measures with the device and kind support for Skope.
- The 3T scanning funds were kindly provided as ‘development time” by the UM faculty of Psychology and Neuroscience.
- The whole brain data in Fig. 2 were acquired with funds from the NWO VENI project 016.Veni.198.032.
- The acquisition of whole brain data shown in Fig. 3B was supported by the BRAIN Initiative (NIH grants R0-MH111444 and U01-EB025162), and NIH R44-MH129278.
- We thank Simon Robinson for discussions about most appropriate coil-combination methods in IcePat for appropriate estimation of Phase data.
- Drs. Poser, Ma, Stirnberg, Stöcker received financial support from the European Union Horizon 2020 Research and Innovation program under grant agreement 885876 (AROMA).
- The sequence work in this abstract was supported through the German Federal Ministry of Education and Research (Bundesministerium für Bildung und Forschung; funding code 01ED2109A) as part of the SCAIFIELD project under the aegis of the EU Joint Programme—Neurodegenerative Disease Research (JPND) (www.jpnd.eu).
- Samantha Ma is supported by Siemens Medical Solutions USA, Inc.
References
- Ahn, C B, and Z H Cho. “A New Phase Correction Method in NMR Imaging Based on Autocorrelation and Histogram Analysis.” IEEE Transactions on Medical Imaging 6, no. 1 (1987): 32–36. https://doi.org/10.1109/TMI.1987.4307795.
- Avants, Brian B., Nicholas J. Tustison, Michael Stauffer, Gang Song, Baohua Wu, and James C. Gee. 2014. “The Insight ToolKit Image Registration Framework.” Frontiers in Neuroinformatics 8. doi: 10.3389/fninf.2014.00044.
- Beckett A, Vu AT, Ahn S, Torrisi S, Polimeni J, Setsompop K, Bilgic B, Stockman J, Feinberg DA. Evaluation of Sub-millimeter resolution in Single-shot EPI on the Next-Generation 7T brain scanner. ISMRM 2022
- Davids M, Dietz P, Ruyters G, Roesler M, Klein V, Guerin B, Feinberg DA, Wald L. PNS optimization of a high-performance asymmetric gradient coil for head imaging. ISMRM, #0565, 2021.
- Feinberg DA, Dietz P, Liu C, Setsompop K Mukherjee P Wald L, Vu A, Beckett A Insua I, Schröder M, Stocker S, Bell Pl, Rummert E, Davids M Design and Development of a Next-Generation 7T human brain scanner with high-performance gradient coil and dense RF arrays. In: ISMRM, #0562, 2021.
- Feinberg DA, Oshio K. Phase errors in multi-shot echo planar imaging. Magn Res Med 1994; 32:535-539.
- Feiweier T, inventor; Siemens Aktiengesellschaft, assignee. Magnetic resonance method and apparatus to determine phase correction parameters. US Patent 8,497,681. July 30, 2013.
- Gunamony S, Feinberg DA An 8-channel transmit 64-channel receive compact head coil for Next Gen 7T scanner with head gradient insert. ISMRM #1447, 2022.
- Heid, Oliver. Method for the phase correction of nuclear magnetic resonance signals, Patent: US006043651A 2000.
- Hoge, W. Scott, and Jonathan R. Polimeni. “Dual-Polarity GRAPPA for Simultaneous Reconstruction and Ghost Correction of Echo Planar Imaging Data.” Magnetic Resonance in Medicine 76, no. 1 (2016): 32–44. https://doi.org/10.1002/mrm.25839.
- Hu, Xiaoping, and Tuong Huu Le. “Artifact Reduction in EPI with Phase-Encoded Reference Scan.” Magnetic Resonance in Medicine 36, no. 1 (July 1996): 166–71. https://doi.org/10.1002/mrm.1910360126.
- Huber L, Poser BA, Bandettini PA, et al. LAYNII: A software suite for layer-fMRI. Neuroimage. 2021;237:118091. doi:10.1016/j.neuroimage.2021.118091
- Jellúš, V. and Kannengießer, S. A. R. (2014). Improved Coil Combination using the Prescan Normalize Adjustment. In SIEMENS Idea meeting 2014, page 4406
- Poser, Benedikt A., Markus Barth, Pål-Erik Goa, Weiran Deng, and V. Andrew Stenger. “Single-Shot Echo-Planar Imaging with Nyquist Ghost Compensation: Interleaved Dual Echo with Acceleration (IDEA) Echo-Planar Imaging (EPI).” Magnetic Resonance in Medicine 69, no. 1 (January 2013): 37–47. https://doi.org/10.1002/mrm.24222.
- Schmitt et al. EPI reconstruction, in Stehling, M K, F Schmitt, and Robert Turner. Echo-Planar Imaging Theory, Technique an Application. Springer-Verlag, 1998.
- Stirnberg R, Stöcker T. Segmented K-Space Blipped-Controlled Aliasing in Parallel Imaging (Skipped-CAIPI) for High Spatiotemporal Resolution Echo Planar Imaging. Magn Reson Med. 2020;85(0):1540-1551. doi:10.1101/2020.06.08.140699
- Stirnberg R, Deistung A, and Stöcker T. T2*-weighted dual-polarity skipped-CAIPI 3D-EPI: 400 microns isotropic whole-brain QSM at 7 Tesla in 6 minutes. #0594, ISMRM, 2022,
- Zwaag, Wietske van der, José P. Marques, Hongxia Lei, Nathalie Just, Tobias Kober, and Rolf Gruetter. “Minimization of Nyquist Ghosting for Echo-Planar Imaging at Ultra-High Fields Based on a ‘Negative Readout Gradient’ Strategy.” Journal of Magnetic Resonance Imaging 30, no. 5 (November 2009): 1171–78. https://doi.org/10.1002/jmri.21951.
Figures
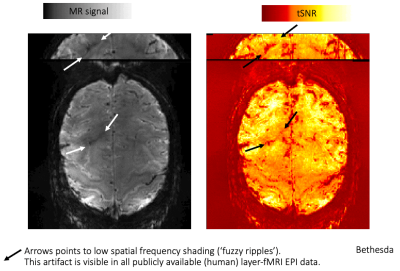
Fig1: Low spatial frequency artifacts (fuzzy ripples) are present in all public and shared layer-fMRI datasets (click on GIF to start animation)
Representative datasets are selected based on public availability and submillimeter resolutions. All data are EPI based (reproduced from layerfmri.com). The purpose of this meta analysis is to convince the reader that low-spatial frequency artifacts are omnipresent in layer-fMRI, and thus, a major challenge for the layer-fMRI community to overcome spatiotemporal resolution limits.
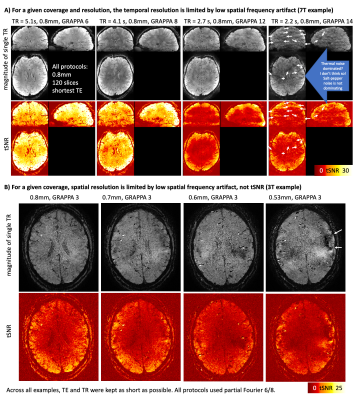
Fig2: Those artifacts prohibit the layer-fMRI community to overcome conventional acquisition limits.
At the scanner, when trying to enhance a conventional layer-fMRI protocol (0.8mm) towards higher spatiotemporal resolutions, images become limited by fuzzy ripples. A) and B) exemplify pushing spatial resolutions with longer EPI trains, and pushing temporal resolutions with more aggressive GRAPPA, respectively. These ‘pushy’ protocols are less limited by SNR (speckle noise) compared to fuzzy ripples (arrows).
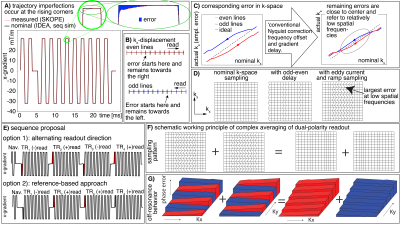
Fig3: Hypothesized origin of the artifact and it’s mitigation strategy: complex averaging with dual polarity EPI
A) Gradient imperfections at the rising corners of trapezoids.
B) With ramp sampling, this results in kx-shifts at mid-spatial frequency representations.
C-D) After phase correction, residual delays remain proximal to k-space center (visualized in two ways).
E) Sequence diagram of proposed dual-polarity EPI.
F-G) Delays and off-resonance effects (Feinberg 1994) are mitigated after complex averaging of dual polarity data.
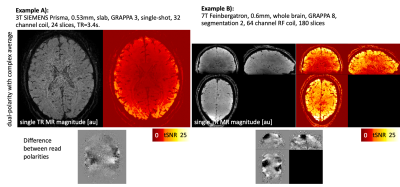
Fig 4: Empirical tests of complex-valued averaging of dual polarity EPI data: It can mitigate the fuzzy ripples (click on GIF to start animation).
Example A) shows that the artifact of Fig. 2B can be fully removed. This allows 3T fMRI at unconventionally high resolutions of 0.53mm.
Example B) shows the artifacts for whole brain fMRI with aggressive 0.63mm and GRAPPA 8. It can be seen that complex averaging of dual-polarity EPI can remove most of it. This allows unconventionally high resolutions and accelerations, with reasonable TRs.
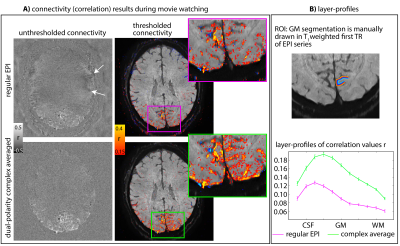
Fig 5: Effect of artifact removal on layer-fMRI connectivity results.
20 min worth of fMRI data during movie watching (Brooklyn Nine-Nine) were used for seed-based correlation analyses. The seed was determined on a manually selected ICA component.
Both rows represent the same raw data (and identical #TRs), reconstructed differently: for conventional EPI and complex-averaged dual-polarity EPI. It can be seen that dual-polarity EPI has stronger connectivity scores while reducing false positives (white arrows).