1140
Feasibility of Highly-accelerated High b-value Multi-shot Diffusion Weighted Imaging by Using Parametric POCSMUSE and Kurtosis Model1The Department of Biomedical Engineering, The Chinese University of Hong Kong, Hong Kong, Hong Kong, 2Multi-scale Medical Robotics Center, Hong Kong, Hong Kong
Synopsis
Keywords: Image Reconstruction, Diffusion/other diffusion imaging techniques, high b-value diffusion weighted imaging, multi-shot diffusion-weighted imaging
High b-value DWI is promising in detection of white matter pathology and infarctions. However, the disadvantages of the acquired high b-value DWI, such as insufficient SNR and image distortions, prohibits its clinical application. Though the feasibility of computed high b-value has been estimated in prostate cancer, the parameters derived from low b-value images cannot be used for diffusion kurtosis model fitting and achieved inferior performance at high b-value. In this study, we proposed a framework based on parametric POCSMUSE and kurtosis model to generate multiple high b-value images with comparable image quality to MUSE from highly-accelerated high b-value DWI.Introduction
High b-value diffusion weighted imaging (DWI) (e.g., b > 1000 s/mm2) can provide better indicators of white matter pathology1 and improve the detection of hyperacute infarction2. However, achieving high-quality high b-value DWI data is challenging because of intrinsically low signal-to-noise ratio (SNR). In addition, the use of single-shot echo planar imaging (ss-EPI) for data acquisition further degrades the image (i.e., geometric distortion) that may hinder the clinical application of high b-value DWI. In light of this, ss-EPI is often combined with SENSE3 or GRAPPA4 to reduce the distortion and improve the spatial resolution, but at the expense of noise amplification. Although multi-shot DW-EPI (ms-DW-EPI) with MUSE5 or POCSMUSE6 can obtain high-quality DW images, the prolonged acquisition time may be impractical for collecting multi-b data. A previous study demonstrated the feasibility of computed high b-value images by using lower b-value images and a mono-exponential model7. However, the parametric models (i.e. intra-voxel incoherent motion (IVIM)8 or diffusion kurtosis imaging(DKI)9) may better approximate the signal change associated with the diffusion properties of the tissues at different b-values. Thus, the integrity of computed high b-value DWI data relies on the use of an appropriate diffusion model. Recently, we have proposed a framework based on parametric POCSMUSE and IVIM model to produce accelerated multi-shot multi-b DW images with comparable quality to fully-sampled MUSE-reconstructed images10. Along this line, we aimed to test the feasibility of producing high-quality high b-value images from undersampled data by using the developed framework with DKI model.Methods
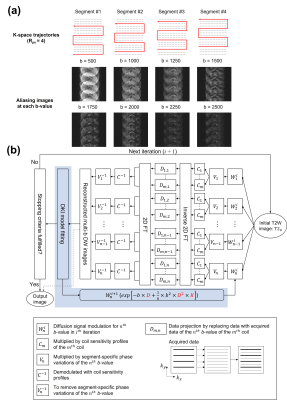
Data acquisition and simulation:The data were collected from one healthy volunteer on a 1.5T MRI scanner (Artist, GE healthcare) using a 12-channel head coil. Two repeated brain DWI datasets were acquired using a 4-shot DW-EPI sequence with 9 b-values (0, 500, 1000, 1250, 1500, 1750, 2000, 2250, 2500 s/mm2). Three orthogonal diffusion directions were acquired for each b-value > 0 with following scan parameters: TE/TR=94/4000ms, matrix size=128x128, slice thickness=5mm, and scantime=6.7mins. Fig.1 shows the comparison between conventional (Fig.1a) and proposed (Fig.1b) sampling trajectory using 4-shot EPI for high b-value DWI acquisition. The fully-sampled 4-shot DW-EPI data was used to simulate an undersampled dataset for each b-value with high in-plane acceleration factor (i.e., Rpe=4), by selecting 1 out of 4 k-space segments (as shown in Fig.2a).
Data reconstruction and evaluation:
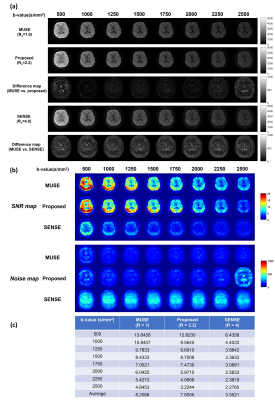
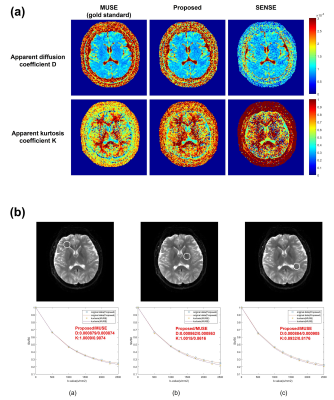
Fig.2b shows the flowchart of parametric POCSMUSE with kurtosis model for simultaneously reconstructing highly-accelerated high b-value images of a single diffusion direction. The fully-sampled b0 image produced by POCSMUSE (S0), and the D and K map (derived from fully-sampled images with b=0/1000/2000 s/mm2) served as initial data at the first iteration. After several iterations, the fitted D and K from the output images with kurtosis model at current iteration were used to update the diffusion-modulation operator ($$$W_n^i$$$ in Fig.2b) for next iteration. The reconstructed high b-value images were the outputs from proposed framework when the iteration converged. Afterward, the image data with three orthogonal diffusion directions were averaged together for producing mean diffusion image for each b-value. The average multi-b DW images produced by MUSE, the proposed method and SENSE were then used for estimating D and K for quantitative estimation. In addition, the fully-sampled and under-sampled high b-value data were respectively reconstructed with MUSE (gold standard) and SENSE for comparison. To evaluate the performance of proposed framework, the effect of SNR in initial b0 image on output multi-b images was explored by adding different noise levels to the POCSMUSE-produced S0 image. Then, the SNR maps for each b-value were measured from a representative slice using the method described in11.
Results
Fig.3 presents the comparison of three reconstruction methods in terms of image difference, SNR and noise maps, and measured SNR values. Fig.4 shows the comparison of high b-value images derived from the proposed framework using the initial images with different noise levels. Fig.5a shows the comparison of D and K maps calculated from three reconstruction methods. Fig.5b displays the curve fitting results with kurtosis model in three selected ROIs from the images reconstructed using either MUSE or the proposed method.Discussion
Our proposed parametric POCSMUSE with kurtosis model can successfully eliminate aliasing artifacts in highly-undersampled high b-value DW images without undesired noise amplification compared to SENSE (Fig.3a). Though the effective scan acceleration factor (Rs) of our proposed method is lower than SENSE, it can achieve comparable SNR to fully-sampled gold-standard MUSE (Figs.3b & 3c) and provide approximate diffusion kurtosis parameters (D and K) to gold standard (Fig.5a). The value of K measured from the images reconstructed by the proposed method is a bit higher compared to gold standard. It is because less signal loss was observed at high b-values within the selected ROIs (Fig.5b). It is noted that the improved SNR performance for high b-value images reconstruction using our proposed method relies on the SNR level of the initial image (Fig.4).Conclusion
The proposed method can produce high b-value multi-shot DW images with reasonable scan time and comparable quality to MUSE-produced images, thereby improving the feasibility of high b-value application using multi-shot DWI.Acknowledgements
The work was in part supported by grants from Hong Kong Research Grant Council (GRF17106820, GRF17125321, and ECS24213522).References
1. Bashat, D.B., V. Kronfeld-Duenias, D.A. Zachor, et al. Accelerated maturation of white matter in young children with autism: a high b value DWI study. Neuroimage. 2007; 37(1): 40-47.
2. Lettau, M. and M. Laible. 3-T high-b-value diffusion-weighted MR imaging in hyperacute ischemic stroke. Journal of Neuroradiology. 2013; 40(3): 149-157.
3. Pruessmann, K.P., M. Weiger, M.B. Scheidegger, and P. Boesiger. SENSE: sensitivity encoding for fast MRI. Magnetic resonance in medicine. 1999; 42(5): 952-962.
4. Griswold, M.A., P.M. Jakob, R.M. Heidemann, et al. Generalized autocalibrating partially parallel acquisitions (GRAPPA). Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine. 2002; 47(6): 1202-1210.
5. Chen, N.K., A. Guidon, H.C. Chang, and A.W. Song. A robust multi-shot scan strategy for high-resolution diffusion weighted MRI enabled by multiplexed sensitivity-encoding (MUSE). Neuroimage. 2013; 72: 41-7.
6. Chu, M.L., H.C. Chang, H.W. Chung, et al. POCS‐based reconstruction of multiplexed sensitivity encoded MRI (POCSMUSE): a general algorithm for reducing motion‐related artifacts. Magnetic resonance in medicine. 2015; 74(5): 1336-1348.
7. Jendoubi, S., M. Wagner, S. Montagne, et al. MRI for prostate cancer: can computed high b-value DWI replace native acquisitions? European Radiology. 2019; 29(10): 5197-5204.
8. Le Bihan, D., E. Breton, D. Lallemand, et al. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology. 1988; 168(2): 497-505.
9. Jensen, J.H., J.A. Helpern, A. Ramani, H. Lu, and K. Kaczynski. Diffusional kurtosis imaging: the quantification of non‐gaussian water diffusion by means of magnetic resonance imaging. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine. 2005; 53(6): 1432-1440.
10. Chen, S., M.-L. Chu, C.-J. Juan, L. Liang, and H.-C. Chang. Highly Accelerated and High-Quality Intra-voxel Incoherent Motion DWI Enabled by Parametric POCS based multiplexed sensitivity-encoding. in International Society for Magnetic Resonance in Medicine (ISMRM) 28th Annual Meeting. 2020. International Society for Magnetic Resonance in Medicine (ISMRM).
11. Gizewski, E.R., S. Maderwald, I. Wanke, et al. Comparison of volume, four-and eight-channel head coils using standard and parallel imaging. European radiology. 2005; 15(8): 1555-1562.
Figures