1139
Quantitative diffusion and Kurtosis MRI in the evaluation of endometrial cancer: validation with histopathology1Physics, CNR Institute for Complex Systems (ISC), Rome, Italy, 2Physics, Sapienza University of Rome, Rome, Italy, 3Radiological, Oncological and Pathological Sciences, Umberto I Hospital, Sapienza University of Rome, Rome, Italy, 4Maternal and Child Health and Urological Sciences, Umberto I Hospital, Sapienza University of Rome, Rome, Italy
Synopsis
Keywords: Pelvis, Cancer, Pelvis, Endometrium, Endometrial cancer, IVIM, Kurtosis
To ameliorate the Endometrial cancer (EC) diagnosis and prognosis, IVIM and KURTOSIS models were used to elaborate DWIs obtained from 18 with EC and 20 healthy women. The b-values were 0,30,50,150,500,800,1000,1500,2000,2500s/mm2. DWIs were noise-corrected considering a homomorphic approach. For each EC subject, ROI in the tumor (T) and peritumoral (PT) area were analysed and endometrial area in healthy (H) subjects was also obtained. IVIM and Kurtosis parameters were quantified. K, which quantifies tissue’s complexity, is significantly higher in T and PT than in H. f is higher in PT compared to the other areas, highlighting the perfusive nature of EC.Introduction
Endometrial cancer (EC) is the second most common gynecological malignancy in Western countries [1]. The diagnosis and preoperative prognosis are essential to perform the needed surgery in the best way for the patient’s well-being and to immediately implement suitable therapy. Current clinical protocols for diagnosing EC are invasive and can lead to various complications [1]. MRI protocols based on ADC and IVIM measurement showed EC-restricted diffusion [2] but the investigations suffer from low specificity and sensitivity. In complex biological tissues such as healthy endometrial and EC, water is hindered by cellular barriers, impeded, and trapped by different biological structures. Therefore, non-Gaussian diffusion models [3,4] could better capture tissue changes due to tumor development. In this work, we test the potential of Diffusion Kurtosis Imaging (DKI) to increase the sensitivity of the EC diagnosis and prognosis by comparing DKI results with the conventional IVIM diffusion model and EC histology.Methods
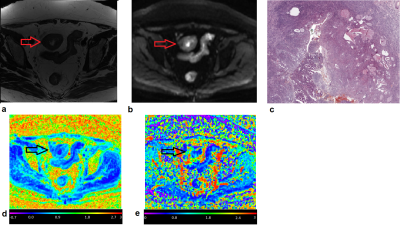
18 volunteers with EC and 20 healthy women were enrolled. The mean (± standard deviation, SD) patient age was 72 (± 10) y and 59 (± 7) y for EC and healthy subjects, respectively. Histological examination was obtained from all the EC subjects. Diffusion-weighted images (DWI) were acquired at 3T using a GE Discovery MR 750 (GE Healthcare, Milwaukee, WI, USA). The acquisition protocol included a Diffusion-weighted Spin-Echo Echo-Planar Imaging with TR/TE=2000ms/77ms; pixel-bandwidth=1953Hz; matrix-size=256x256, FOV=300x300 mm2, number of slices = from 9 to 31. The in-plane resolution was 1.2x1.2mm2 and the slice thickness was 5mm with GAP =6mm. The diffusion encoding gradients were applied along 3 no-coplanar directions using ten different b-values (0,30,50,150,500,800,1000,1500,2000,2500 s/mm2). The number of averaged signals (NSA) for each b-value was NSA=2. Since the acquisitions were done implementing an unknown accelerator algorithm, each DWI was noise corrected considering a homomorphic approach [3]. A machine learning algorithm based on bugged trees was used in order to obtain parametric MRI maps of the diffusion coefficient D in a non-Gaussian environment and of the kurtosis coefficient K, which quantifies the deviation from the Gaussian behavior (Figure 1). For each subject with EC, ROI in the tumor area (T) and the area immediately around the tumor area (peritumoral, PT) were analyzed separately. A healthy endometrial ROI area (H) was considered in healthy subjects. In each zone, the mean value of the diffusion parameters obtained from: a) IVIM model (f, and ) and b) the kurtosis model (K and D) [4] [5] were obtained. The bi-exponential IVIM model was performed by fixing obtained from a mono-exponential fitting performed at b = (500,800,1000,1500 s/mm2). Differences between means were analyzed using a Kruskal-Wallis test with Dunn and Sidák’s post-hoc correction and Cohen’s d effective size.Results
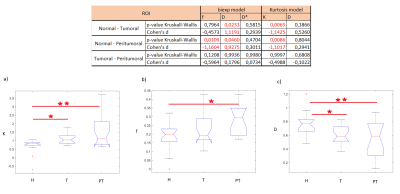
The kurtosis K was higher in PT and T ROIs than in H ROIs. The f parameter was higher in the PT compared to its value in the H ROIs, whereas D values were lower in the T and the PT ROIs than in the healthy ROI H (see Figure 2).Discussion
K obtained in T and PT is significantly higher than that obtained in H (Figure 2). K parameter quantifies the tissue's complexity, which increases in cancer tissues compared to H tissues as confirmed by histology. Furthermore, the IVIM f parameter calculated in PT is significantly higher than that obtained in H (Figure 2). This could be related to a higher percentage of perfusion in PT zones where the tumor infiltrates healthy tissues. The diffusion coefficient D is higher in healthy tissues than on peritumoral and tumoral tissues characterized by more complex structures.Conclusion
K and f provide important information on the tumor contour and on a possible expansion of the tumor. Consequently, K could optimize the prognosis of endometrial cancer. Moreover, the IVIM model with fixed D also describes the experimental data trend, which identifies the perfusive nature of endometrial cancer.Acknowledgements
No acknowledgement found.References
[1] P. Morice, A. Leary, C. Creutzberg, N. Abu-Rustum and E. Darai, “Endometrial cancer,” Lancet, vol. 387, p. 1094–1108, 2016.
[2] S. Satta, M. Dolciami, V. Celli, F. Di Stadio, G. Perniola, I. Palaia, A. Pernazza, C. Della Rocca, S. Rizzo, C. Catalano, S. Capuani and L. Manganaro, “Quantitative diffusion and perfusion MRI in the evaluation of endometrial cancer: validation with histopathological parameters,” The British Journal of Radiology, vol. 94, no. 1125, 2021.
[3] S. Aja-Fernández, T. Pieciak and G. Vegas-Sánchez-Ferrero, “Spatially variant noise estimation in MRI: A homomorphic approach,” Medical Image Analysis, vol. 20, p. 184–197, 2015.
[4] J. H. Jensen, J. A. Helpern, A. Ramani, H. Lu and K. Kaczynski, “Diffusional Kurtosis Imaging: The Quantification of Non-Gaussian Water Diffusion by Means of Magnetic Resonance Imaging,” Magnetic Resonance in Medicine, vol. 53, pp. 1432-1440, 2005.
[5] J. Jensen and J.A. Helpern, “MRI quantification of non-Gaussian water diffusion by kurtosis analysis,” NMR Biomed, vol. 23, pp. 698-710, 2010.
[6] V. G. Kiselev, “Fundamentals of diffusion MRI physics,” NMR in Biomedicine, vol. 30, 2017.
Figures