1135
Measuring water exchange in myelinated white matter using Magnetization Transfer (MT)-weighted constant gradient diffusion MRI (MT-cgdMRI)1Department of Radiology, NYU Grossman School of Medicine, New York, NY, United States, 2Vilcek Institute of Graduate Biomedical Sciences, NYU Grossman School of Medicine, New York, NY, United States, 3NYU Grossman School of Medicine, New York, NY, United States
Synopsis
Keywords: White Matter, Contrast Mechanisms, Water exchange
In white matter, myelin forms a barrier for water exchange between the intra-axonal and extra-axonal compartments. Although myelin is not directly visible in conventional diffusion MRI (dMRI), it may affect dMRI measurements via exchange. In this study, we compared two ways to combine magnetization transfer (MT) preparation with constant gradient dMRI (cg-dMRI) to study the effects of myelin on water exchange rate. Our results demonstrate that MT preparation can modulate the sensitivity of cg-dMRI to exchange by suppressing signals from a portion of exchanging spins and that placing MT preparation is more effective before diffusion encoding than during diffusion encoding.Introduction
Water exchange between compartments in the brain is a critical biological process for maintaining homeostasis and its non-invasive measurement may serve as a biomarker for diagnosis of structural and functional deficits1. Within myelinated white matter (WM), there are multiple exchange processes among tissue compartments, such as exchange between intra-axonal, extra-axonal and myelin water2,3. Several MRI methods that utilize differences in diffusivities or relaxivities among those compartments to measure exchanges have been introduced4-8. As the myelin sheath forms a physical barrier between the intra and extracellular compartments, models have been introduced that consider it (together with myelin water) as an intermediate compartment for water exchange3,7. In diffusion MRI (dMRI), myelin and myelin water are mostly invisible due to their short T2s, but their effect on dMRI signals can still be detected3,9.In this study, we introduced an MT preparation (off-resonance RF pulses)10 to constant gradient (cg) dMRI measurement6 to suppress myelin water signals and examined how this affected the estimation of water exchange rate in myelinated WM.
Methods
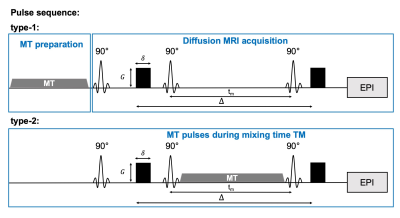
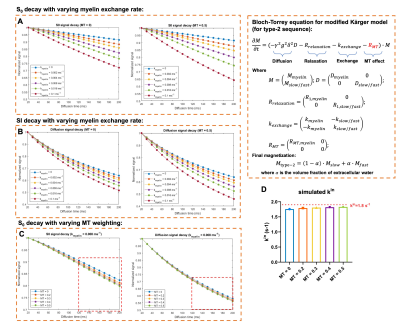
All experimental procedures were approved by the Institutional Animal Care and Use Committee. Data were acquired from inbred BALB/cJ mouse brains (n=5, perfusion fixed) at room temperature. Two types of MT-weighted cg-dMRI sequences were proposed here to probe water exchange (Fig 1): 1) The type-1 sequence placed the MT preparation before diffusion encoding (200 Gaussian pulses, bandwidth=1.5kHz, offset frequency=3kHz, peak amplitude=4/8/16μT); 2) The type-2 sequence placed the MT preparation during diffusion encoding by varying the number of pulses. Other imaging parameters are: one-shot STEAM-EPI, matrix size =128×96, resolution = 0.1×0.1 mm2, 1.5mm slice thickness, TR/TE = 3000/35ms, δ= 4ms, q2= 0/15/30/45/60/89/104/118 x103 mm-2, Δ range from 35ms to 200ms (130 to 200 ms for type-2). The diffusion direction was applied perpendicular to axons in the corpus callosum. Exchange rate ($$$k_{in}$$$) was calculated from cg-dMRI signals with q2=118 x103 mm-2 with the T1 effect removed as described in6. Numerical solution was performed to type-2 sequence based on Block-Torrey equation for modified Karger model. The effect of MT preparation was inserted as a relaxation term (RMT), assuming the effect is linear with the length of MT preparation pulse train. Other parameters were obtained from previous studies.Results
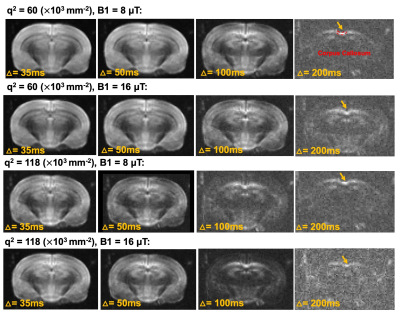
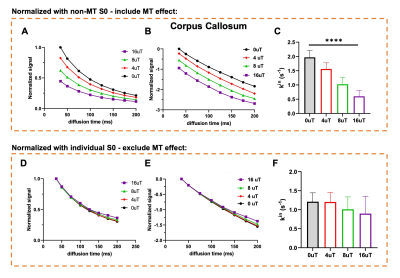
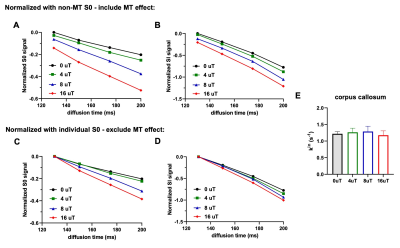
Fig. 2 shows representative dMRI images with q2 = 60 and 118x103 mm-2 and peak B1 amplitude of 8 and 16 μT. Even at high q2, long Δ, and strong MT preparation, the signals from the corpus callosum remained above the noise floor. For type-1 sequence, adding MT introduced additional signal attenuation (Fig. 3A-B). Without considering MT-related attenuation, the estimated $$$k_{in}$$$ decreased with increasing MT B1 amplitude (Fig. 3C). When MT-related attenuation was excluded by normalizing with non-diffusion-weighted signals at each MT B1 amplitude, the estimated $$$k_{in}$$$ only showed slight decrease with increasing MT B1 amplitude. For type-2 sequence, adding MT also introduced additional signal attenuation (Fig. 4A-D), and the estimated $$$k_{in}$$$ showed an initial increase with MT B1 amplitude but decreased at high amplitude (16 μT). This observation was supported by numerical solution of Karger model (Fig. 5).Discussion
The addition of MT preparation to cg-dMRI does not alter the actual water exchange rate but only makes a portion of the exchanging spins MR-invisible before or during diffusion encoding (Fig1). As a result, the estimated $$$k_{in}$$$ from cg-dMRI without considering MT-related attenuation is reduced (Fig. 3C). As dMRI-based exchange measurements have been limited to the slow exchange regime, adding MT preparation can potentially allow us to evaluate slightly faster exchange processes. Placing MT-preparation during diffusion encoding (type-2) is more complex than placing it before diffusion encoding (type-1) as the exchanging spins will undergo simultaneous MT saturation, T1 relaxation, and diffusion/exchange. Furthermore, the length of MT-preparation in this case is limited by the range of diffusion time and can only saturation a smaller number of exchanging spins than type-1 sequence.Conclusion
MT preparation can modulate the sensitivity of cg-dMRI to exchange by suppressing signals from a portion of exchanging spins.Acknowledgements
The project was supported by the National Institute of Health (NIH) R01 HD 074593, R01 NS 102904 and R01 NS088040, and was performed using shared resource supported byNIH 1S10OD018337-01, 5P30CA016087, and P41 EB017183.References
1. Springer, C. S. Using (H2O)-H-1 MR to measure and map sodium pump activity in vivo. Journal of Magnetic Resonance 291, 110-126, doi:10.1016/j.jmr.2018.02.018 (2018).
2. Stanisz, G. J., Szafer, A., Wright, G. A. & Henkelman, R. M. An analytical model of restricted diffusion in bovine optic nerve. Magnet Reson Med 37, 103-111, doi:DOI 10.1002/mrm.1910370115 (1997).
3. Brusini, L., Menegaz, G. & Nilsson, M. Monte Carlo Simulations of Water Exchange Through Myelin Wraps: Implications for Diffusion MRI. IEEE Trans Med Imaging 38, 1438-1445, doi:10.1109/TMI.2019.2894398 (2019).
4. Harkins, K. D., Dula, A. N. & Does, M. D. Effect of intercompartmental water exchange on the apparent myelin water fraction in multiexponential T2 measurements of rat spinal cord. Magnet Reson Med 67, 793-800, doi:10.1002/mrm.23053 (2012).
5. Lasic, S., Nilsson, M., Latt, J., Stahlberg, F. & Topgaard, D. Apparent Exchange Rate Mapping with Diffusion MRI. Magnet Reson Med 66, 356-365, doi:10.1002/mrm.22782 (2011).
6. Meier, C., Dreher, W. & Lebrfritz, D. Diffusion in compartmental systems. I. A comparison of an analytical model with simulations. Magnet Reson Med 50, 500-509, doi:10.1002/mrm.10557 (2003).
7. van Gelderen, P. & Duyn, J. H. White matter intercompartmental water exchange rates determined from detailed modeling of the myelin sheath. Magnet Reson Med 81, 628-638, doi:10.1002/mrm.27398 (2019).
8. Fieremans, E., Novikov, D. S., Jensen, J. H. & Helpern, J. A. Monte Carlo study of a two-compartment exchange model of diffusion. Nmr in Biomedicine 23, 711-724, doi:10.1002/nbm.1577 (2010).
9. Lee, H. H., Novikov, D.S., Fieremans, E. in ISMRM. 0839.
10. van Zijl, P. C. M., Lam, W. W., Xu, J., Knutsson, L. & Stanisz, G. J. Magnetization Transfer Contrast and Chemical Exchange Saturation Transfer MRI. Features and analysis of the field-dependent saturation spectrum. Neuroimage 168, 222-241, doi:10.1016/j.neuroimage.2017.04.045 (2018).
Figures