1119
Novel 3D Quantitative Magnetization Transfer Imaging of The Whole Knee Cartilage1Athinoula A. Martinos Center for Biomedical Imaging, Harvard Medical School, Charlestown, MA, United States, 2Radiology, Massachusetts General Hospital, Boston, MA, United States, 3Orthopedic Surgery, Massachusetts General Hospital, Boston, MA, United States
Synopsis
Keywords: Cartilage, Magnetization transfer
We propose to characterize cartilage extracellular matrix of the whole knee using a novel quantitative magnetization transfer (MT) imaging technique. This new technique achieves accurate T1 and MT quantification by using a new sequence incorporating off-resonance RF pulses that create two simultaneous effects: 1) Bloch-Siegert phase shift to measure and correct for B1+ inhomogeneity and 2) direct saturation of macromolecules to model MT. This new method, termed BTS (Bloch-Siegert and magnetization Transfer Simultaneously), is presented and validated in both phantom and in-vivo knee imaging experiments.Introduction
The extracellular matrix of articular cartilage consists of water and small concentrations of macromolecules which include type II collagen and proteoglycan1,2. Quantitative magnetization transfer (qMT) provides parametric maps of macromolecule bound proton fraction (f), a measure of macromolecule content, and exchange rate between free water and macromolecule bound protons (kF)3, a measure of macromolecule integrity. While a previous study has found that qMT parameters are sensitive to cartilage degeneration in Osteoarthritis4, the observation is only shown in patellar cartilage due to the limited imaging capability of covering the whole knee. Recently, we have developed a new method named BTS (Bloch-Siegert and magnetization Transfer Simultaneously)5 to obtain bias-free T1 and qMT parameters with large volume coverage. This new method leverages an off-resonance RF pulse in an RF-spoiled gradient-echo sequence to simultaneously encode Bloch-Siegert6 shift to correct B1+-induced errors and induce macromolecule saturation to model magnetization transfer effects7. Here, we implement a BTS protocol to image the whole knee and investigate the feasibility of this new approach for characterizing the whole knee cartilage extracellular matrix.Theory
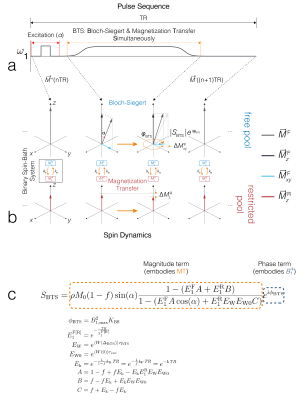
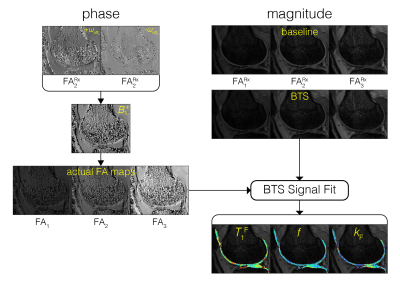
The BTS method is based on an RF-spoiled gradient-echo scheme. Off-resonance irradiation, whose frequency is significantly larger than its peak amplitude and Larmor frequency (ωoff ≫ ω1max, ωo), is introduced between excitation and acquisition (FIG1A). Adding off-resonance RF induces two independent effects: 1) MT through direct saturation of macromolecules and 2) B1+ dependent Bloch-Siegert phase shift6 of free water protons. Utilizing the binary spin-bath system, the Bloch-McConnell equations can be solved in the steady-state to obtain an analytical signal equation (FIG1C), whereby MT is encoded through signal magnitude and B1+ through signal phase. Using a variable flip-angle (vFA) scheme with (BTS) and without (baseline) off-resonance RF applied, these signal terms are used to generate a B1+ map, T1F (MT-corrected free water T1) map, and MT parameters f and kF maps.Methods
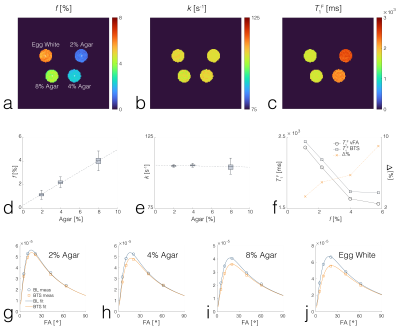
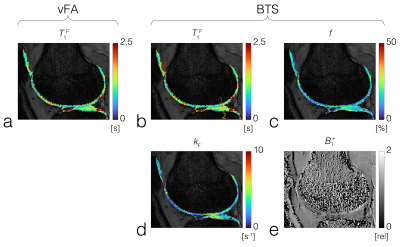
BTS was validated through phantom and in-vivo knee experiments on a 3T scanner (Siemens Prisma). A 20-channel head coil was used for the phantom experiment, and a 4-channel flex coil was used for the in-vivo experiment. Phantom experiment: Phantoms consisted of four 20mL vials emersed in a 200μM MnCl2 water bath. Vials consisted of 2%, 4%, 8% agar (weight per volume) dissolved in DI water and boiled egg white. Common 3D parameters for both BTS and baseline acquisition: matrix size = 128x128x12 yielding 1.3x1.3x5mm resolution, TE/TR = 12/80ms, gradient spoiling moment = 600mT·ms/m, RF spoil increment = 169˚, and prescribed flip-angles (FAs) 5˚, 10˚, 20˚, 40˚, 60˚. For BTS acquisitions, an 8ms fermi MT saturation pulse with off-resonance frequency 4000Hz and effective flip-angle 786˚ was used, assuming a Gaussian absorption line shape3. In-vivo experiment: Common 3D parameters for both BTS and baseline acquisition: matrix size = 224x192x36 yielding 0.7x0.7x3mm resolution along the sagittal plane, TE/TR = 12/70ms, gradient spoiling moment = 320mT·ms/m, RF spoil increment = 169˚, and prescribed flip-angles 10˚, 20˚, 40˚. MT saturation pulse with effective flip-angle 629˚ was applied for BTS acquisitions assuming a Super-Lorentzian absorption line shape8. An in-vivo knee scan was performed on a healthy subject. Inter-scan motion was compensated using the co-registration method9. The phase term of the BTS equation was used to estimate B1+6 and subsequently used to calculate the actual flip-angle maps to compensate transmitting field inhomogeneity. These were then applied to the magnitude term of the BTS equation to extract T1F, f, and kF, assuming fixed MT properties for macromolecule bound proton T1R = 1s and T2R = 12μs (FIG 2).Results and Discussion
Phantom experiment: Results show f increases linearly with agar concentration (FID3A,D), while the fundamental rate constant (=kF/f) was relatively constant (FID3B,E), in agreement with previous results3. T1F estimated from vFA showed a significant difference with that estimated from BTS (FIG3C), whereby this difference increased with f(FIG3F). Representative baseline and BTS fit curves taken from voxels in each compartment, indicated by white circles (FIG3A), show good agreement with measured data (FIG3G-J). In-vivo experiment: T1F obtained using vFA (FIG4A) and BTS (FIG4B), and MT parameters f (FIG4C) and kF (FIG4D) obtained from BTS were measured in the femoral, tibial and patellar cartilage ROI regions shown as numbered dotted rectangles in FIG4B. T1F (vFA), T1F (BTS), f and kF for femoral cartilage (1):0.829s[s]/0.903[s]/12.5[%]/1.92[s-1]; tibial cartilage (2): 1.007[s]/1.050[s]/12.4[%]/2.41[s-1]; patellar cartilage (3): 1.015[s]/1.133[s]/12.9[%]/1.78[s-1], in agreement with literature4,10.Conclusion
A new qMT method leveraging simultaneous estimation of B1+ dependent phase and MT effect through off-resonance RF has been introduced. Phantom experiments demonstrated that T1 estimated using normal vFA can be significantly biased by MT effects which increases with increased macromolecular content, whereas T1 estimated from BTS is independent of MT effects and also insensitive to transmitting field inhomogeneity. The proposed BTS method can image the whole knee successfully and provide 3D B1+- and MT-corrected T1 and qMT maps, including f and kF for joint cartilage. Spatial variation and regional features of these BTS-derived parameter maps on knee cartilages showed good agreement with the previous literatures4,10. Therefore, BTS presents great potential to provide new bias-corrected relaxometry and qMT imaging biomarkers to characterize cartilage extracellular matrix in the whole knee.Acknowledgements
We thank the funding support from NIBIB R21EB031185, NIAMS R01AR079442 and R01AR081344.References
1. Crema MD, Roemer FW, Marra MD, Burstein D, Gold GE, Eckstein F, Baum T, Mosher TJ, Carrino JA, Guermazi A. Articular cartilage in the knee: current MR imaging techniques and applications in clinical practice and research. Radiographics 2011;31:37-61.
2. Choi JA, Gold GE. MR imaging of articular cartilage physiology. Magn Resonance Imaging Clin N Am 2011;19:249-282.
3. Henkelman RM, Huang X, Xiang Q, Stanisz GJ, Swanson SD, Bronskill MJ. Quantitative Interpretation of Magnetization Transfer. Magn Reson Med 1993;29:759-766.
4. Sritanyaratana N, Samsonov A, Mossahebi P, Wilson JJ, Block WF, Kijowski R. Cross-relaxation imaging of human patellar cartilage in vivo at 3.0T. Osteoarthritis Cartilage 2014;22:1568-1576.
5. Jang A, Han PK, Ma C, El Fakhri G, Liu F. Bias-free T1 Mapping via Simultaneously Estimating Bloch-Siegert and Magnetization Transfer Effects. ISMRM 2022, London, UK.
6. Sacolick LI, Wiesinger F, Hancu I, Vogel MW. B1 Mapping by Bloch-Siegert Shift. Magn Reson Med 2010;63:1315–1322.
7. Wolff SD, Balaban RS. Magnetization Transfer Contrast (MTC) and Tissue Water Proton Relaxation in Vivo. Magn Reson Med 1989;10:135-144.
8. Morrison C, Henkelman RM. A Model for Magnetization Transfer in Tissues. Magn Reson Med 1995;33:475-482.
9. Klein S, Staring M, Murphy K, Viergever MA, Pluim JPW. elastix: A Toolbox for Intensity-BasedMedical Image Registration. IEEE Trans Med Imaging 2010;29(1):196-205.
10. Liu F, Block WF, Kijowski R, Samsonov A. Rapid Multicomponent Relaxometry in Steady State with Correction of Magnetization Transfer Effects. Magn Reson Med 2016;75:1423-1433.
Figures