1106
MR-STAT for fast contrast agent uptake quantification1Computational Imaging Group for MR diagnostics & therapy, Center for Image Sciences, UMC Utrecht, Utrecht, Netherlands, Utrecht, Netherlands
Synopsis
Keywords: Quantitative Imaging, Contrast Agent, MR-STAT; Contrast Enhancement Imaging
Gadolinium-based contrast agents (GBCAs) uptake has the ability to facilitate disease diagnosis. In this work, we implement MR-STAT for fast contrast agent uptake quantification by applying keyhole acquisition and regularized reconstruction. We first analyze the accuracy of the proposed method on gadolinium-doped gel phantoms and observe that accurate T1 mapping of post-injection can be achieved with a keyhole factor of 25%. Furthermore, we investigate the impact of lower (20% vs 100%) GBCA dose administration on simulated clinical data. The quantification of pathologic T1 change for low-dose administration was comparable to that for full-dose.Introduction
Gadolinium-based contrast agents (GBCAs) are widely used to alter the relaxation properties of the pathologic tissue1 to facilitate disease diagnosis. Recently, there has been a clear need to reduce the dose of GBCAs due to safety issues and concerns about gadolinium deposition2, water pollutant3, and imaging cost4. The relationship5 between GBCAs concentration and T1/T2 values can be leveraged to reduce the amount of administered contrast agent, provided that the changes in T1/T2 in the pathologic tissue are clearly quantifiable.MR-STAT is a quantitative MRI technique used to map multiple parameters (e.g. T1, T2, PD) simultaneously from a five-minute, full-brain scan. When quantifying T1 and/or T2 to detect the changes in pathologic tissues between pre and post GBCA-injection, there is no need to run two full MR-STAT acquisitions; only the local contrast information changes significantly upon injecting GBCA. Therefore, we can drastically under-sample the post-injection acquisition considering that the additional information is mainly encoded in the low spatial frequency components in k-space. In this work, we first analyze the accuracy of the accelerated acquisition using the quantitative MR-STAT framework on gadolinium-doped gel phantoms at 1.5T. Afterwards, we investigate the impact of lower (20% vs 100%) GBCA dose administration in detecting T1 changes by testing simulated data derived from a clinical brain tumor trial8.
Methods
The flowchart of the keyhole MR-STAT method is illustrated in Figure 1. The proposed acquisition/reconstruction procedure consists of the following five steps.Step 1: Full pre-injection and under-sampled post-injection MR-STAT acquisitions;
step 2: pre-injection high-resolution MR-STAT reconstruction9 of (T1, T2, ρ);
step 3: Regularized10 low-resolution reconstruction of the post-injection (T1, T2, ρ);
step 4: Fourier domain combination of pre-injection and post-injection quantitative maps where the central region of the k-space (contrast information) consists of the low-resolution post-injection maps;
step 5: the high-resolution post-injection maps are finally obtained by transforming to image space.
T1 maps
Considering the primary effect of GBCAs on T1 shortening, we focus our analysis on T1 changes.
Phantom experiment
Quantitative MRI studies were performed on 1.5T MR scanner (Ingenia, Philips Healthcare, Best, The Netherlands). 7 Eurospin gel tubes and a tube consisting of gelatin dissolved in tap water and gadobutrol (1.0 M Gadovist, Schering AG, Berlin, Germany) at a concentration of 0.2 mM were prepared to simulate a “pre-injection” phantom (7 Eurospin gel tubes mimic the healthy tissue, the gadolinium-doped tube mimic the tumor tissue). After the “pre-injection” scan, the “tumor” tube was replaced by one with a gadobutrol concentration of 0.5 mM to simulate a “post-injection” acquisition. Data were acquired from a single axial slice using a Cartesian MR-STAT sequence11. Imaging parameters were: slice thickness: 3 mm; FOV: 224×224 mm2; The pre-injection acquisition takes 10 s per slice. We investigate three post-injection MR-STAT acquisitions: a full one (10 s duration), a 50% keyhole (5 s), and a 25% keyhole (2.5 s). See Figure 2.
In-vivo simulated experiment
We retrospectively selected MR-STAT clinical data8 from two tumor patients, the lesions of which are segmented by a neuro-radiologist in training, and used these data as pre-injection data. Subsequently, we calculated the T1 and T2 values in tumor areas of post-injection with known R1 and R2 relaxivities12 for two gadobutrol concentration (C) levels (full (100%) and low-dose (20%)) by following the equations5: 1/T1post= 1/T1pre + R1·C; 1/T2post= 1/T2pre + R2·C. The simulated post-injection signals are generated by assuming a post-injection acquisition with a keyhole factor of 25%.
Results
Figure 3 shows T1 maps and the correlation between MR-STAT measurements and the ground truth from fully and under-sampled data of post-injection. Post-injection T1 maps from under-sampled data were similar to those generated from the fully sampled data and the change in the T1 value of the “tumor” tube is very clear.Each pre-injection, low-dose post-injection, and full-dose post-injection T1 map of simulated clinical data from two tumor patients are shown in Figure 4 and Figure 5. The quantification of pathologic T1 change for low-dose administration was comparable to that for full-dose (bottom row of Figures 4 & 5).
Discussion and Conclusion
We implemented and evaluated MR-STAT for fast contrast agent uptake quantification of gadolinium on phantoms and simulated clinical data. The results demonstrate that accurate T1 mapping of post-injection can be achieved with a keyhole factor of 25% (equivalent to approximately 1-minute acquisition time for the whole brain). In addition, results from simulated clinical brain data with different doses of contrast agent suggest that pathological changes can be detected even with administration of lower (20%) dosage contrast agents.In future work, prospective in vivo validation will also be performed. Possibly, the quantification of T1 also provides the opportunity to quickly detect the time-dependent concentration of contrast agents, and high 2D temporal resolution in keyhole MR-STAT acquisition (=2.5 s) is therefore adequate for 2D Dynamic contrast-enhanced MRI13.
Acknowledgements
This
work has been financed by the Netherlands Organisation for Scientific Research
(NWO), HTSM Grant 17986. The authors are grateful to Ms Sarah M. Jacobs for
segmenting the tumor lesions in the two patient datasets.
References
1. Brasch RC, Weinmann HJ, Wesbey GE. Contrast-enhanced NMRimaging: animal studies using gadolinium-DTPA complex. Am J Roentgenol. 1984;142(3):625–630.
2. McDonald RJ, McDonald JS, Kallmes DF, et al. Gadolinium deposition in human brain tissues after contrast-enhanced MR imaging in adult patients without intracranial abnormalities. Radiology. 2017, 285(2): 546-554.
3. Rogowska J, Olkowska E, Ratajczyk W, Wolska L. Gadolinium as a new emerging contaminant of aquatic environments. Environ Toxicol Chem. 2018;37(6):1523- 1534.
4. Shankar PR, Parikh K, Davenport MS. Financial implications of revised ACR guidelines for estimated glomerular filtration rate testing before contrast- enhanced MRI. J Am Coll Radiol. 2018;15(2):250- 257.
5. Lauffer RB. Paramagnetic metal complexes as water proton relaxation agents for NMR imaging: theory and design. Chem Rev. 1987;87(5):901-927.
6. Sbrizzi A, van der Heide O, Cloos M, et al. Fast quantitative MRI as a nonlinear tomography problem. Magn Reson Imaging. 2018;46:56–63.
7. van Vaals JJ, Brummer ME, Dixon WT, Tuithof HH, Engels H, Nelson RC, Gerety BM, Chezmar JL, den Boer JA. "Keyhole" method for accelerating imaging of contrast agent uptake. J Magn Reson Imaging. 1993;3(4):671–675.
8. Kleinloog JPD, Mandija S, D'Agata F, Liu H, van der Heide O, Koktas B, Jacobs SM, van den Berg CAT, Hendrikse J, van der Kolk AG, Sbrizzi A. Synthetic MRI with Magnetic Resonance Spin TomogrAphy in Time-Domain (MR-STAT): Results from a Prospective Cross-Sectional Clinical Trial. J Magn Reson Imaging. 2022 Sep 13. doi: 10.1002/jmri.28425.
9. Liu H, Heide OV, Mandija S, Berg CATV, Sbrizzi A. Acceleration strategies for MR-STAT: Achieving high-resolution reconstructions on a desktop pc within 3 minutes. IEEE Trans Med Imaging. 2022; 41(10): 2681-2692.
10. Park M Y, Hastie T. L1‐regularization path algorithm for generalized linear models. Journal of the Royal Statistical Society: Series B (Statistical Methodology). 2007, 69(4): 659-677.
11. Fuderer M, van der Heide O, Liu H, Berg CATV, Sbrizzi A. Efficient performance analysis and optimization of transient-state sequences for multi-parametric MRI. NMR in Biomedicine. 2022, (in press)
12. Pintaske J, Martirosian P, Graf H, et al. Relaxivity of gadopentetate dimeglumine (Magnevist), gadobutrol (Gadovist), and gadobenate dimeglumine (MultiHance) in human blood plasma at 0.2, 1.5, and 3 Tesla. Investigative radiology. 2006, 41(3): 213-221.
13. Khalifa F, Soliman A, El‐Baz A, et al. Models and methods for analyzing DCE‐MRI: A review. Medical physics. 2014, 41(12): 124301.Figures
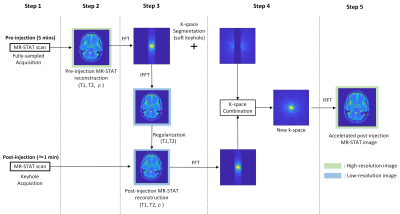
Figure 1. Flowchart of the proposed keyhole MR-STAT method.
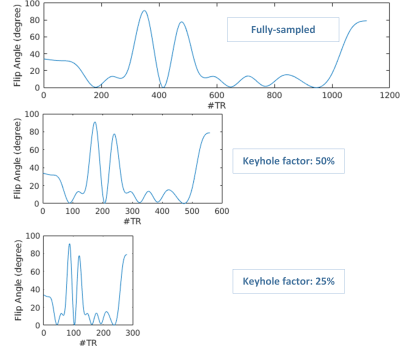
Figure 2. Flip angle train scheme used in the keyhole MR-STAT acquisition.
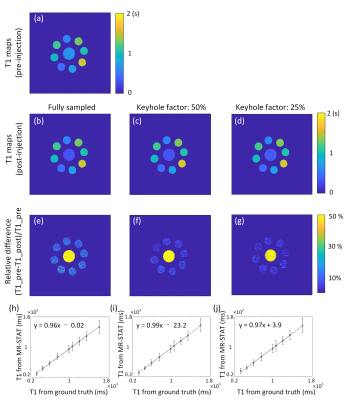
Figure 3. In vitro validation of gadolinium-altered MR-STAT measurements. (a) – (d) Pre and post-injection T1 map acquired with fully sampled and keyhole factor of 25% and 50%; (e)- (g) The relative difference images between pre-acquisition T1 and post-acquisition T1 : (h)-(j) Comparison and linear regression analysis between MR-STAT measurements and the ground truth of post-injection. Each data point represents measurements from a gel-tube. Error bars represent the standard deviation in the corresponding gel tubes.
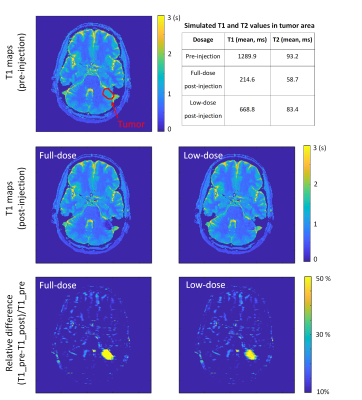
Figure 4. T1 maps of simulated clinical data from patient A with different dosages of contrast agent by applying the keyhole MR-STAT method (keyhole factor 25%). The values in the table are the ground truth of the simulated “pre- and post-injection” in the tumor area.
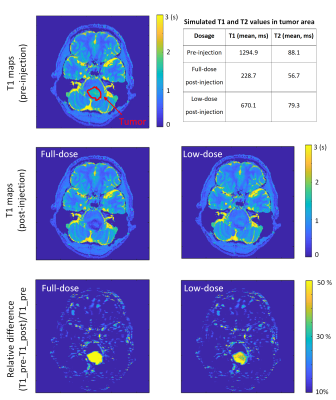
Figure 5. T1 maps of simulated clinical data from patient B with different dosages of the contrast agent by applying the keyhole MR-STAT method (keyhole factor 25%). The values in the table are the ground truth of the simulated “pre- and post-injection” in the tumor area.