1093
Towards isotropic 3D whole-heart T1 mapping using model-based motion-corrected super-resolution reconstruction1Physikalisch-Technische Bundesanstalt (PTB), Braunschweig and Berlin, Germany, 2Charité Medical Faculty University Medicine, Berlin, Germany, 3Working Group on Cardiovascular Magnetic Resonance, Experimental and Clinical Research Center (ECRC), Charité Humboldt University Berlin, DZHK partner site Berlin, Berlin, Germany, 4Department of Cardiology and Nephrology, HELIOS Klinikum Berlin Buch, Berlin, Germany, 5School of Biomedical Engineering and Imaging Sciences, King’s College London, London, United Kingdom, 6Department of Biomedical Engineering, Technical University of Berlin, Berlin, Germany
Synopsis
Keywords: Quantitative Imaging, Heart, Super-Resolution Reconstruction
Cardiac T1 mapping provides valuable information for the diagnosis of a variety of heart diseases. However, due to SNR and scan time limitations, often only 2D imaging with a low through-plane resolution covering a few slices of the left ventricle is possible. In this work, a super-resolution reconstruction approach is presented aiming towards whole-heart 1.3 mm isotropic T1 mapping within less than three minutes acquisition time. The proposed approach provided a whole-heart cardiac T1 map including the atria and the right ventricle with improved visualization of small structures and overall image quality.
Introduction
Cardiac T1 mapping provides valuable information for the diagnosis of a variety of heart diseases1–3. In clinical routine, usually only 2D T1 maps with low through-plane resolution are acquired. However, isotropic high-resolution whole-heart coverage including atria would strongly improve diagnostic information about fibrosis in arrhythmic patients4.So far, whole-heart T1 mapping with high isotropic resolution (below 1.5 mm) is only possible with long acquisition times (>9 minutes)5–8. In this work, a super-resolution reconstruction (SRR) approach is presented, aiming towards whole-heart cardiac T1 mapping with 1.3 mm isotropic resolution in 2.8 minutes acquisition time.
Methods
As shown in Figure 1, rotated stacks of T1 maps were acquired for the proposed approach (SRRrot) which is in contrast to previous SRR approaches (SRRtransl) that shifted the 2D stacks relative to one another9. Motion-corrected model-based SRR9 was applied to calculate a 3D high-resolution T1 map from the 2D stacks.Data acquisition
The approach was evaluated using simulations, phantom scans and in vivo data.
In vivo and phantom data was acquired using a 3 Tesla MRI (Verio, Siemens Healthineers, Erlangen, Germany) with a 32-channel cardiac coil. Slices were acquired continuously with a Golden radial sampling scheme for 2.8 seconds per slice: flip angle α: 5°, resolution: (1.3×1.3×8.0) mm³, inversion pulse: slice-selective. For comparison, a 3(3)3(3)5 modified Look-Locker Inversion Recovery (MOLLI) sequence was acquired: TE/TR: 1.1/2.6 ms, flip angle: 35°, spatial resolution: 2.2×1.5×8 mm3. For the phantom scans, the T1MES phantom10 was used and reference scans were acquired orthogonal to the original scan orientation.
Data acquisition with the above parameters was simulated using anatomy models from XCAT11.
For SRR, 12 stacks consisting of five parallel slices each were acquired with a slice gap of 14 mm. Every stack was acquired in a 14 seconds breath hold. The stacks had the same positions but were rotated (0°, 30°, 60°, 90°, 120°, 150°) around the phase-encoding direction (PE). The septum was aligned parallel to PE, so the stacks were rotated from a four-chamber view (4CH) over a two-chamber view (2CH) back in direction of 4CH. Two stacks always shared the same orientation and were shifted by 11 mm to one another to fill the slice gaps.
For comparison purpose, SRRtransl was acquired according to 9, so all stacks were oriented in short-axis view (SAX) but shifted to one another by 1.8 mm along the slice-encoding direction.
Motion-corrected model-based SRR
As proposed in 9, non-rigid cardiac motion and translational residual respiratory motion in between breath holds were estimated and used for SRR.
The SRR was model based, so the spatial resolution model and T1-relaxation model were integrated into the calculation of the 3D T1 map. For minimization of this optimization problem, the difference between the acquired low-resolution dynamics and the ones predicted from the high-resolution T1 map was minimized. The optimization problem was initialized using a combination of all stacks and solved using an alternating optimization scheme12.
Evaluation
For the simulations, the obtained SRR T1 maps were compared to the ground truth maps. For the phantom scans, T1 values were assessed in manually selected regions-of-interest (ROIs) in each of the nine tubes. A bull’s eye plots analysis13 was carried out for the in vivo scans.
Results
Figure 2 shows the results using simulated data. In the same scan time, the proposed approach could visualize the whole heart, while SRRtransl was limited to the ventricles. The visualization of small structures such as the right ventricle improved.The application of SRRrot to phantom data improved the overall visualization of the tubes compared to SRRtransl (Figure 3). The accuracy of the T1 values evaluated in ROIs over all tubes increased by 8.38% in SRRrot compared to SRRtransl (difference to reference: SRRrot 13.91 ± 13.36 ms, SRRtransl 15.18 ± 14.28 ms).
Figure 4 compares SRR applied to in vivo data with a MOLLI scan. The proposed approach could provide T1 maps of the whole heart, including the atria. In the same acquisition time, SRRtransl could only cover the ventricles.
Figure 5 shows a bull’s eye plot evaluation of the proposed approach. SRRrot provides comparable T1 precision as SRRtransl, as indicated by the low T1 standard deviation within the myocardial segments. Magnetization transfer effects lead to a small underestimation of the T1 values using SRR, due to the use of a slice selective inversion pulse14.
Discussion
With the proposed approach, the whole heart could be covered. The visualization of small structures and the accuracy of estimated T1 values improved using the proposed approach, compared to previously proposed SRR approaches. The use of a slice-selective inversion pulse led to apparently short T1 times of the blood due to inflow effects, which improved the visualization of the atrial wall as it increased the contrast between the fine structures of the atria and blood.In future approaches, a consideration of intra-stack motion and a fully affined motion model for the breath hold alignment might improve the overall SRR result.
Conclusion
The proposed SRR approach provided an isotropic 3D high-resolution whole-heart cardiac T1 map including the atria and the right ventricle in less than three minutes. The overall image quality and visualization of small structures improved.Acknowledgements
The authors gratefully acknowledge funding from the German Research Foundation (GRK2260, BIOQIC). The results presented here have been developed in the framework of the 18HLT05 QUIERO Project. This project has received funding from the EMPIR programme co-financed by the Participating States and from the European Union’s Horizon 2020 research and innovation programme.References
1. Haaf P, Garg P, Messroghli DR, Broadbent DA, Greenwood JP, Plein S. Cardiac T1 Mapping and Extracellular Volume (ECV) in clinical practice: a comprehensive review. J Cardiovasc Magn Reson. 2016;18(1):89. doi:10.1186/s12968-016-0308-4
2. Schelbert EB, Messroghli DR. State of the Art: Clinical Applications of Cardiac T1 Mapping. Radiology. 2016;278(3):658-676. doi:10.1148/radiol.2016141802
3. Al-Wakeel-Marquard N, Seidel F, Herbst C, et al. Diffuse myocardial fibrosis by T1 mapping is associated with heart failure in pediatric primary dilated cardiomyopathy. Int J Cardiol. 2021;333:219-225. doi:10.1016/j.ijcard.2021.03.023
4. Beinart R, Khurram IM, Liu S, et al. Cardiac magnetic resonance T1 mapping of left atrial myocardium. Heart Rhythm. 2013;10(9):1325-1331. doi:10.1016/j.hrthm.2013.05.003
5. Phair A, Cruz G, Qi H, Botnar RM, Prieto C. Free‐running 3D whole‐heart T 1 and T 2 mapping and cine MRI using low‐rank reconstruction with non‐rigid cardiac motion correction. Magn Reson Med. 2023;89(1):217-232. doi:10.1002/mrm.29449
6. Qi H, Jaubert O, Bustin A, et al. Free‐running 3D whole heart myocardial T 1 mapping with isotropic spatial resolution. Magn Reson Med. 2019;82(4):1331-1342. doi:10.1002/mrm.27811
7. Qi H, Bustin A, Kuestner T, et al. Respiratory motion-compensated high-resolution 3D whole-heart T1ρ mapping. Journal of Cardiovascular Magnetic Resonance. 2020;22(1):12. doi:10.1186/s12968-020-0597-5
8. Milotta G, Bustin A, Jaubert O, Neji R, Prieto C, Botnar RM. 3D whole‐heart isotropic‐resolution motion‐compensated joint T 1 /T 2 mapping and water/fat imaging. Magn Reson Med. 2020;84(6):3009-3026. doi:10.1002/mrm.28330
9. Hufnagel S, Metzner S, Kerkering KM, et al. 3D model-based super-resolution motion-corrected cardiac T1 mapping. Phys Med Biol. Published online October 20, 2022:0-23. doi:10.1088/1361-6560/ac9c40
10. Captur G, Gatehouse P, Keenan KE, et al. A medical device-grade T1 and ECV phantom for global T1 mapping quality assurance — the T 1 Mapping and ECV Standardization in cardiovascular magnetic resonance ( T1MES ) program. Journal of Cardiovascular Magnetic Resonance. Published online 2016:1-20. doi:10.1186/s12968-016-0280-z
11. Segars WP, Sturgeon G, Mendonca S, Grimes J, Tsui BMW. 4D XCAT phantom for multimodality imaging research. Med Phys. 2010;37(9):4902-4915. doi:10.1118/1.3480985
12. Bano W, Piredda GF, Davies M, et al. Model‐based super‐resolution reconstruction of T 2 maps. Magn Reson Med. 2020;83(3):906-919. doi:10.1002/mrm.27981
13. Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized Myocardial Segmentation and Nomenclature for Tomographic Imaging of the Heart. Circulation. 2002;105(4):539-542. doi:10.1161/hc0402.102975
14. Huang L, Neji R, Nazir MS, et al. FASt single‐breathhold 2D multislice myocardial T 1 mapping (FAST1) at 1.5T for full left ventricular coverage in three breathholds. Journal of Magnetic Resonance Imaging. 2020;51(2):492-504. doi:10.1002/jmri.26869
Figures
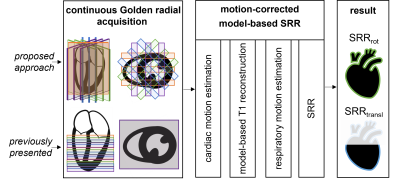
Figure 1: Workflow of the proposed approach. Data was acquired using a continuous Golden-angle radial acquisition. Previously presented approaches9 acquired all stacks in short-axis view and shifted them to one another (SRRtransl). In the proposed approach (SRRrot), the stacks were acquired at the same position but with different orientations. The motion-corrected model-based SRR9 was then applied to the datasets, allowing whole-heart coverage for SRRrot while SRRtransl was limited to the ventricles.

Figure 2: SRR applied on simulated data. The T1 map using the proposed approach (SRRrot) is compared to SRR using translations (SRRtransl) and the ground truth. In the same scan time, the proposed approach could visualize the whole heart, including the atria (pink arrow), while SRRtransl was limited to the ventricles. Next to that, the visualization of small structures such as the right ventricle improved in SRRrot (red arrow) compared to SRRtransl. Voxels presenting blood were simulated with a low apparent T1 value, due to the use of a slice selective inversion pulse.
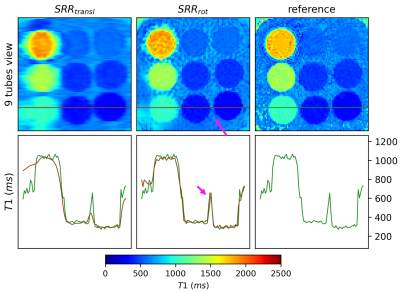
Figure 3: Super-resolution reconstruction (SRR) applied to T1MES phantom data. The T1 map resulting from the proposed approach (SRRrot) is compared to the output from SRR using only translations (SRRtransl), next to an orthogonal acquisition, serving as a reference. A line plot through three tubes is shown, once for the reference in green and for SRRtransl and SRRrot in brown. In the same scan time, the overall image quality improved using SRRrot compared to SRRtransl, as can be seen, for example, in the improved distinction between tubes and background (pink arrow).
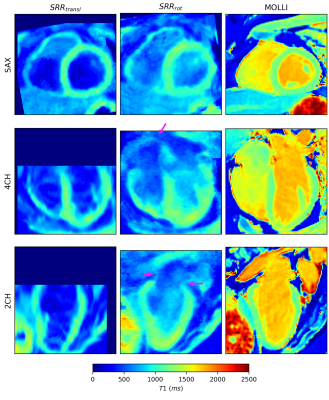
Figure 4: The application of the proposed approach on in vivo data. A short-axis (SAX), four-chamber (4CH) and two-chamber (2CH) view of the SRR T1 maps are compared to a MOLLI reference scan. SRRrot describes the T1 maps resulting from the proposed approach, while SRRtransl describes the output from SRR using only translation. SRRrot covered the whole myocardium efficiently, including the atria (pink arrow). In the same acquisition time, SRRtransl was limited to the ventricles. Voxels presenting blood in SRR have a low T1 value, due to the use of a slice selective inversion pulse.
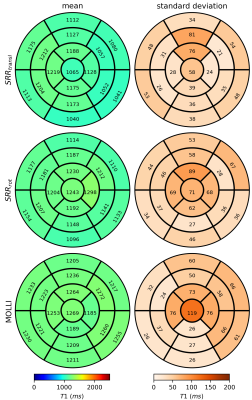
Figure 5: Bull's eye evaluation. The mean and standard deviation of the T1 values in myocardial segments resulting from the proposed approach (SRRrot ) are compared to the ones from super-resolution reconstruction (SRR) using only translations (SRRtransl) and the MOLLI reference scans. Both SRR approaches provided precise T1 maps. Small underestimation of the T1 values compared to MOLLI can be attributed to magnetization transfer effects14.