1086
Investigation of the effect of deep-breathing on the caval circulation in patients with cardiac rhythm disorders using 5D radial Flow MRI
Sara Boccalini1, Marta Beghella2, Loic Boussel1,2, Philippe Douek2,3, Philippe Chevalier4, Claudia Prieto5, and Monica Sigovan6
1Department of Radiology, HCL, Lyon, France, 2CREATIS, Lyon, France, 3Departement of Radiology, HCL, Lyon, France, 4Department of Cardiac Rythm, HCL, Lyon, France, 5School of Biomedical Engineering and Imaging Sciences, King’s College London, London, United Kingdom, 6CNRS, CREATIS Lab, Lyon, France
1Department of Radiology, HCL, Lyon, France, 2CREATIS, Lyon, France, 3Departement of Radiology, HCL, Lyon, France, 4Department of Cardiac Rythm, HCL, Lyon, France, 5School of Biomedical Engineering and Imaging Sciences, King’s College London, London, United Kingdom, 6CNRS, CREATIS Lab, Lyon, France
Synopsis
Keywords: Quantitative Imaging, Velocity & Flow
Respiratory motion effects thoracic blood flow mainly by intrathoracic pressure changes. In addition, deep-inspirations strongly increase the systemic venous return with immediate repercussions on right ventricular stroke volume. Simultaneously, pulmonary resistance is increased leading to decreased inflow in the left chambers, which is compensated in the following heartbeats. In patients with atrial myopathy (ex. atrial fibrillation), these adjustments might be more difficult. Our aim was to assess the impact of deep breathing on hemodynamic parameters in patients with atrial fibrillation, paroxysmal and permanent, and in a healthy population as compared to normal breathing.INTRODUCTION
Thoracic blood flow is based on a complex relationship between different physiological systems, mainly heart and respiratory pump. The respiratory pump system provides blood flow mainly by intrathoracic pressure changes.Conventional assessment of thoracic blood flow using three-dimensional cardiac-resolved (4D) phase contrast MRI (PC-MRI) is not suitable for the assessment of the respiratory pump effect because standard pencil beam respiratory gating is performed in only one specific respiratory phase. To overcome this limitation, free-running self-gated non-cartesian 4D Flow MRI acquisitions have been developed. Ma et. al. demonstrated recently the feasibility of assessing the respiratory related changes in the caval circulation under physiological breathing conditions using a 5D radial phase contrast acquisition with compressed sensing reconstruction1.
It is known that deep inspirations increase strongly the systemic venous return with immediate repercussions on right ventricular stroke volume. At the same time, pulmonary resistance is increased leading to decreased inflow in the left chambers, which will be compensated in the following heartbeat. In patients with atrial myopathy, such as in the presence of atrial fibrillation, all these quick adjustments might be more difficult or not possible. Although a few studies have explored alterations of hemodynamic parameters on in the left atrium in patients with atrial fibrillation, the effect of deep breathing has not yet been assessed.
Therefore, our aim was to assess the impact of deep breathing on hemodynamic parameters in patients with atrial fibrillation, paroxysmal and permanent, and in a healthy population as compared to normal breathing.
METHODS
We included 4 patients with paroxysmal AF, 4 patients with permanent AF, and 5 age matched healthy volunteers. MR imaging included standard of care cardiac function assessment using 2D cine bSSFP sequences and a free-running 3D radial Flow acquisition that we implemented on a 1.5T Philips Ingenia system (Philips, Best, The Netherlands)2. The 3D radial trajectory is based on a spiral phyllotaxis pattern for k-space sampling adapted from Piccini et al.3. The acquisition was interleaved with 8 projections per interleaf and performed with the following parameters: TE/TR 2.5/6.0 ms, flip angle = 6◦, VENC = 70 cm/s, FOV = 340x340x340 mm3, 2.5 -2.7 mm isotropic voxel size, acquisition time 8 -12 minutes.Blood flow measurements were performed twice: once under physiological respiration, i.e. normal breathing (NB) and repeated under forced respiration, i.e. deep-breathing (DB) when subjects were asked to breathe in and breathe out deeply during data acquisition.
The respiratory self-gated (SG) signal was derived using a sliding window reconstruction method we proposed recently4. Briefly, 64 consecutive spokes with a step of 8 spokes are used to yield high temporal and low spatial resolution velocity resolved volumes. In this work, we obtained the 1D translation of the lung/liver interface from each volume using an ROI defined on the fully-sampled reconstruction. The obtained self-gating respiratory signal was then used to bin the deep-breathing acquisition in 3 respiratory phases. The normal breathing acquisition was not respiratory resolved to ensure comparable respiratory amplitudes. Subsequently, each respiratory phase (3 DB and 1 NB) was binned in 8 cardiac phases using the ECG signal and a fixed temporal resolution. This strategy reduces the velocity errors related to averaging over variable RR durations. Respiratory- and cardiac-resolved images were then reconstructed offline using a compressed sensing algorithm implemented in Matlab (The Mathworks, Inc, Natick, MA) using first order finite differences as sparsifying transforms in space and time, for both cardiac and respiratory dimensions. The right atrium (RA), superior vena cava and inferior vena cava (IVC) were manual segmented on time-averaged phase contrast angiography (TAVG-PCMRA) of each individual respiratory phase. Cardiac and respiratory resolved average velocities and flows were obtained for the two caval veins.
RESULTS
All datasets were reconstructed successfully. Nevertheless, breathing artefacts were present for strong respiratory amplitudes (> 3 cm of diaphragm excursion). Subjects presented relatively different deep-breathing patterns with average amplitudes per respiratory bin varying between 1.4 to 4.6 cm. Representative time-averaged speed MIP images of the right atrium (RA) and the caval veins (SVC and IVC) are presented in Figure 1. Displacement of the RA due to respiratory motion is clearly visible between DB inspiration and DB expiration phases. Representative flow patterns for a healthy volunteer and a patient with a history of AF are presented in Figure1. The respective intra-bin respiratory amplitudes (cm) are presented in the figure. Respiratory and cardiac resolved average speed measurements for the same patients are presented in Figure 2. Increased velocities were observed in the SVC and decreased velocities in the IVC for DB-Inspiration phase. Oppositely, decreased velocities were observed in the SVC and increased velocities in the IVC for DB-Expiration phase.DISCUSSION
We demonstrate the feasibility of investigating blood flow changes in the thoracic caval circulation under deep-breathing conditions. While challenging due to the very high respiratory amplitudes proved promising to explore the previously disregarded effect of respiration on cardiac physiology, in particular systemic venous return.Preliminary results demonstrate respiratory related changes in blood flow in healthy subjects with the most evident variations on the SVC. These changes appeared less pronounced in patients with atrial fibrillation. The proposed method may potentially refine diagnosis in several heart diseases, including congenital heart disease.
Acknowledgements
Funding: ANR-18-CE19-0025-01References
1. Ma, L. E. et al. 5D Flow MRI: A Fully Self-gated, Free-running Framework for Cardiac and Respiratory Motion–resolved 3D Hemodynamics. Radiol. Cardiothorac. Imaging, e200219 (2020).
2. Sigovan, M. et al. Self-gated respiratory-resolved 5D Flow MRI using the 3D spiral phyllotaxis trajectory. in ISMRM (2018)
3. Piccini, D. et al, “Spiral phyllotaxis: the natural way to construct a 3D radial trajectory in MRI”, Magnetic Resonance in Medicine 66:1049–1056 (2011)
4. Boccalini, S. et al, Investigation of Left-Atrial flows using a 3D radial based self-gated respiratory motion corrected 4D Flow MRI sequence. in ESMRMB (2021).
Figures
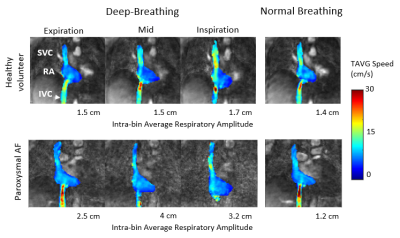
Representative
results for a healthy volunteer (top) and a paroxysmal AF patient (bottom). Respiratory
resolved time average (TAVG) speed MIP images (jet color scale) of the right
atrium (RA) and caval veins (superior vena cava (SVC) and inferior vena cava (IVC))
overlaid on TAVG PCMRA images (gray scale) acquired from left to right: in DB expiration,
DB mid, DB inspiration, and NB. Increased velocities in the SVC are clearly
visible in the DB Inspiration phase for both subjects.
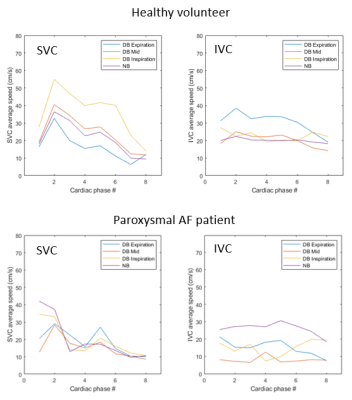
Cardiac resolved and respiratory resolved average speed
measured in the superior vena cava (SVC) and the inferior vena cava (IVC) for the
healthy volunteer (top) and the paroxysmal AF patient (bottom) presented in
figure 1.
DOI: https://doi.org/10.58530/2023/1086