1069
Cardiac Library Based Real-time Imaging (CALIBRI): towards 3D cardiac visualization during MR-guided cardiac interventions1Department of Radiology & Nuclear Medicine, Amsterdam UMC, Amsterdam, Netherlands, 2Department of Biomedical Engineering & Physics, Amsterdam UMC, Amsterdam, Netherlands, 3Department of Cardiology, Amsterdam UMC, Amsterdam, Netherlands
Synopsis
Keywords: Arrhythmia, MR-Guided Interventions, Real-time imaging
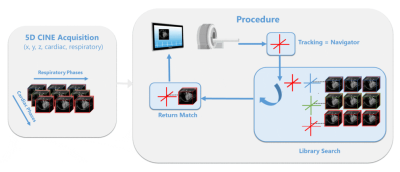
We propose Cardiac Library Based Real-time Imaging (CALIBRI), which allows the use of the same data for both catheter tracking and real-time visualization of cardiac motion states. This is achieved by matching the acquired k-lines with a library of pre-acquired cardio/respiratory gated 3D CINE data. In this study, we implemented our novel method on a 3T MRI system and showed the feasibility of this approach in multiple healthy volunteers.Introduction
Interventional cardiac magnetic resonance (iCMR) has the potential to become a powerful platform to guide complex cardiac interventions [1,2]. In contrast to X-ray guidance, MRI allows for radiation-free, combined 3D visualization of anatomy and precise device localization. Real-time MR is, however, rather slow compared to conventional fluoroscopy frame rates (i.e. 15 frames/s for standard fluoroscopy) [3]. Although active catheter tracking can be performed at very high frame rates using only a small set of 3 orthogonal k-lines, simultaneous 3D visualization of the heart remains highly unfeasible, as heavily undersampled 3D CINE techniques require computationally demanding iterative reconstruction techniques [4,5,6].Here, we propose a novel framework, called Cardiac Library Based Real-time Imaging (CALIBRI), which allows the use of the same data for both catheter tracking and real-time visualization of cardiac motion states. This is achieved by matching the acquired k-lines with a library of pre-acquired cardio/respiratory gated 3D CINE data (Fig.1).To test feasibility, this novel method was implemented on a 3T MRI system and applied in healthy volunteers.
Theory
CALIBRI (Fig.1) utilizes the assumption that each cardiac (and respiratory) motion state provides unique features in a single or very limited set of k-lines. As illustrated by Fig.2, periodic k-space variations match the corresponding ECG signal and the heart rate is clearly visible in the Fourier spectra of, almost, all individual spatial frequencies. This concept has already been utilized by studies [4,7] for self-gating purposes. More importantly, the abundance of information within single k-lines suggests that a single navigator can serve as a heart phase specific signature. In other words, by continuously acquiring such data during a so-called tracking sequence, the corresponding motion state could be derived in real-time. In case of an active catheter tracking sequence that uses three orthogonal navigator lines for device localization, 3D cardiac motion information can be visualized at a temporal resolution of only 3 TRs provided that the library search is sufficiently fast.Methods
Acquisition and Reconstruction: Data was acquired in 3 healthy volunteers on a 3T scanner (Ingenia/Elition, Philips, Best, NL). To build the patient-specific library of cardiac images, free-breathing whole-heart 3D bSSFP data was acquired using a Cartesian pseudo-spiral k-space trajectory [8]. Specific imaging parameters were: TR/TE=2.9/1.45 ms, flip angle = 45°, FOV = 320x160x160 mm, resolution = 2x2x2 mm3, acquisition time = 5-6 min. Subsequently, the same sequence was performed for 1D tracking by acquiring k0 lines in the read-out direction for approximately 2 minutes. For reconstruction, 3D CINE scans were retrospectively gated to 15 cardiac and 4 respiratory phases (5D-data) based on measured ECG and the respiratory signals and using compressed sensing with BART [9] (acceleration factor R≅9).Library search: For demonstration, the library was generated using the center k-lines of the 3D CINE (volunteer 1) and from part of the tracking dataset (volunteer 2+3) as the groundtruth ECG is available to link with the 3D CINE. First, a principle component analysis was performed on the tracking data to select the optimal coil for the library search. Then, for each tracking line, the correlation in the form of an inner product was calculated with all corresponding k0-lines from the 5D library. The tracking line with the highest correlation provided the corresponding cardiac phase. (Note that this search also finds the corresponding respiratory phase.)
Results and Discussion
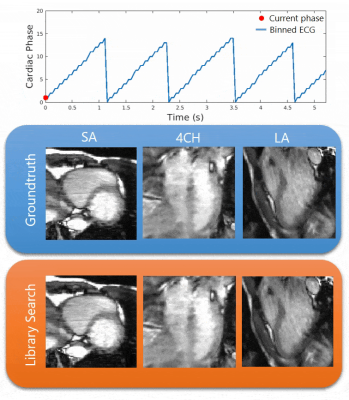
Quantitative results of the library search for all scans are shown in Fig.3. The left two columns show 3D/2D histograms of the agreement between the cardiac phase chosen as the best match versus the reference cardiac phase as determined from the ECG. The right column shows the correlations based upon which the best library match was chosen per cardiac phase, which are typically all > 0.99. While the 3D histograms show less successful matching in diastole, correlations remain comparable to the successful matches in systole. This probably results from the fact that in diastole, both the ventricles and atria are stationary and therefore, the cardiac phase is less unique. Notably, we use the ECG to generate the ground truth cardiac phases, even though this signal is not always reliable due to continuous rapid switching gradients, resulting in an incorrect registration of the R-top [10].Fig.4 visualizes qualitative CALIBRI results (volunteer 2) comparing real-time cardiac motion obtained from the library search in comparison with the groundtruth, in the orange and blue panels, respectively. The visualization supports the results of the correlation plots in Fig.2; even though the matching is not perfect in the diastole quantitatively, the visual effect on cardiac motion remains minimal and should therefore provide sufficient information for real-time cardiac interventions.
Conclusion and Outlook
With these initial results, we have shown the feasibility of CALIBRI as a promising technique for real-time 3D cardiac visualization during MR-guided interventions. In our future work, we will combine the tracking sequence with real-time catheter tracking for visualization of devices during 3D cardiac motion. Furthermore, we foresee numerous options to improve the library search algorithm using advanced signal processing or AI based algorithms.Acknowledgements
No acknowledgement found.References
[1] Bhagirath P, van der Graaf M, Karim R et al. Interventional cardiac magnetic resonance imaging in electrophysiology: advances toward clinical translation. Circ Arrhythm Electrophysiol. 2015 Feb;8(1):203-11
[2] Campbell-Washburn AE, Tavallaei MA, Pop M, et al. Real-time MRI guidance of cardiac interventions. J Magn Reson Imaging. 2017 Oct;46(4):935-950.
[3] Dzavik V. Impact of Low Frame Rate Fluoroscopy and Cine-angiography on Reducing Operator and Patient Dose. ClinicalTrials.gov. Identifier: NCT02574949; October 2015
[4] Lorenzo Di Sopra, Piccine D, Coppo S et al. An automated approach to fully self-gated free-running cardiac and respiratory motion-resolved 5D whole-heart MRI. MRM. 2019 May 24;(145(4):1422-1435. Doi: 10.1093/brain/awab411.
[5] Usman M, Ruijsink B, Nazir MS, et al. Free breathing whole –heart 3D CINE MRI with self-gated Cartesian trajectory. Magn Reson Imaging. 2017 May;38:129-137
[6] Moghari MH, Barthur A, Amaral ME, et al. Free-breathing Whole-heart 3D Cine Magnetic Resonance Imaging with Prospective Respiratory Motion Compensation. Magn Reson Med. 2019 Jul;80(10):181-189
[7] Han F, Rapacchi S and Hu P. Improved cardiac motion self-gating. Journal of Cardiovasular Magnetic Resonance. 2012. 15, Article number: P83
[8] Merton R, Schrauben EM, Strijkers GJ, et al. Reproducibility of aortic diameter and displacement derived from free-breathing 3D balanced steady-state free precession CINE images at 3T. ISMRM Proceedings London 2022. https://archive.ismrm.org/2022/1229.html.
[9] Uecker, M. & Tamir, J., et al. mrirecon/bart: version 0.5.00. (2019) doi: 10.5281/ZENODO.3376744
[10] Fischer SE, Wickline SA and Lorenz CH. Novel real-time R-wave detection algorithm based on the vectorcardiogram for accurate gated magnetic resonance acquisitions. Magn Reson Med. 1999 Aug;42(2):361-70.
Figures