1068
A modular sparse hemispherical transmit/receive phased array for microbubble-mediated MR-guided focused ultrasound brain therapy1Sunnybrook Research Institute, Toronto, ON, Canada, 2University of Toronto, Toronto, ON, Canada
Synopsis
Keywords: New Devices, Focused Ultrasound
MRI is commonly employed to assess microbubble-mediated focused ultrasound (FUS) treatment outcomes, but is restricted to post-treatment evaluations due to its low temporal resolution. Here, we describe a modular sparse hemispherical transmit/receive phased array for microbubble-mediated FUS brain therapy and simultaneous 3D passive cavitation imaging (PCI) for real-time intraoperative treatment monitoring and control. The device was evaluated via MR-guided FUS experiments in rabbits. The utility of 3D PCI in calibrating FUS exposure levels and predicting MRI-inferred tissue damage volume distributions resulting from high target level sonications was demonstrated.Introduction
Focused ultrasound (FUS) applied in conjunction with circulating microbubbles can induce a wide range of biological effects on brain vasculature and tissue in vivo1,2. MRI is commonly used to assess microbubble-mediated FUS treatment outcomes1,2, however the low temporal resolution of MRI (acquisition times ≈ seconds-to-minutes) relative to the timescales over which the relevant ultrasound-microbubble-vessel interactions take place (microseconds-to-milliseconds), restricts the use of MRI to post-treatment evaluations in this context. 3D passive cavitation imaging (PCI) with large-aperture 2D receiver arrays can localize microbubble activity in the brain during FUS3-6, and spatially-coherent acoustic emissions have been employed as an internal calibration metric for FUS exposure control5,6. Here, we evaluate 3D PCI for exposure calibration and treatment monitoring during microbubble-mediated FUS brain therapy using a novel modular sparse hemispherical transmit/receive phased array.Methods
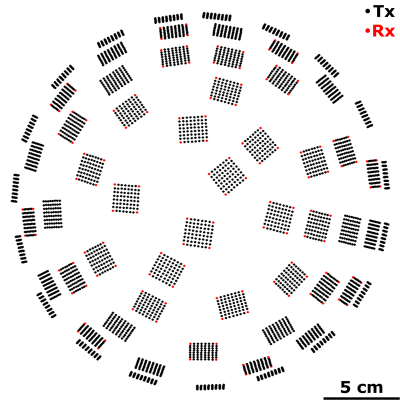
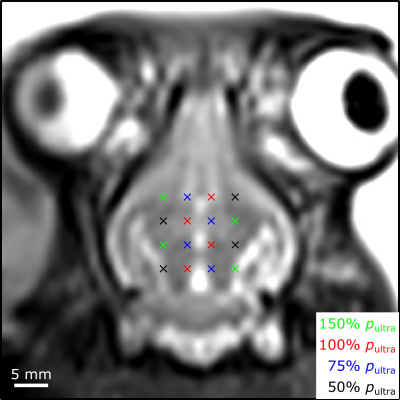
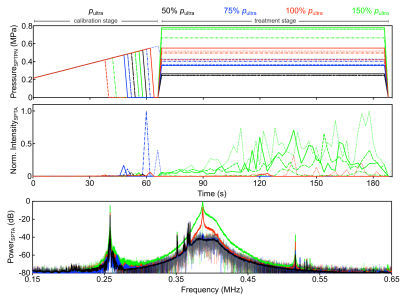
System Design: The phased array comprises 64 flat transducer modules with a half-wavelength inter-element spacing7,8. Each module consists of 64 square ceramic elements (width = 2.2 mm) arranged in an 8 x 8 square grid (center-to-center spacing = 2.5 mm). The 60 non-corner elements were employed as transmitters, driven at their lateral mode resonant frequency (f = 258 kHz). The 4 corner elements were employed as receivers, and were tuned near the first ultraharmonic (1.5 x f = 387 kHz) of the driving frequency (Fig.1). Ultrasound field simulations were carried out to optimize the spatial distribution of the transducer modules. The MRI-compatible transducer array and driving system were designed to mount on the standard bed of a 3T MRI scanner (Biograph mMR, Siemens Healthcare, Erlangen, Germany).FUS: Experiments were performed on New Zealand White rabbits (male, 3-4 kg). Burst-mode FUS (3,840 transmit elements, f = 258 kHz, pulse length = 10 ms, pulse repetition frequency = 0.5 Hz, duration = 120 s) was steered electronically over 12-16 point grids (axial plane, 3.5 mm point spacing, see Fig.2) following microbubble injection (4 μl/kg DefinityTM) via online multi-point 3D PCI-based exposure level calibration5,6 (128 receiver elements, whole-burst temporal averaging). Exposures were carried out at 50-150% of the pressure required to detect ultraharmonic activity (pultra) in vivo (Fig.3).
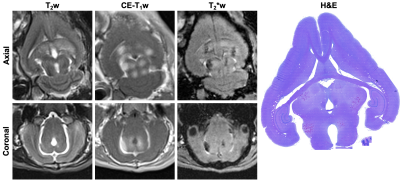
MRI: T2-weighted (T2w) MRI scans (3D SPACE spin echo; TR: 5000 ms, TE: 240 ms, slice thickness: 1.5 mm) were acquired pre-treatment for targeting purposes. Post-treatment, contrast-enhanced T1-weighted (CE-T1w) MRI scans (turbo spin echo; TR: 500-700 ms, TE: 8.6 ms, slice thickness: 1.5 mm) were acquired to detect increases in blood-brain barrier (BBB) permeability and T2*-weighted (T2*w) MRI scans (3D gradient-echo; TR: 27 ms, TE: 15 ms, slice thickness: 1.5 mm) were acquired to monitor for red blood cell (RBC) extravasations produced by the exposures1. Animals were sacrificed within 6 hours of sonication for histopathological examination (hematoxylin and eosin (H&E) stained tissue sections).
Results
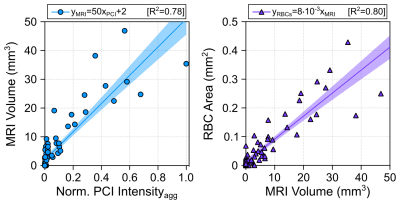
Spatially-coherent ultraharmonic activity was detected trans-rabbit skull near the intended target locations during pressure ramp sonications (0.7 ± 0.3 MPa, free-field estimate). CE-T1w MRI signal enhancement was detected at all target levels immediately post-sonication, indicative of increased levels of BBB permeability (Fig.4). T2*w MRI revealed signal hypointensities induced by all exposures at p ≥ 100% pultra, occasional exposures at p = 75% pultra, and no exposures at p = 50% pultra (Fig.4). The tissue damage volumes assessed via T2*w MRI were found to increase with increasing target level, and were associated with regions of RBC extravasations on H&E sections (Fig.4, Fig. 5). Sonication-aggregate 3D PCI data generated via delay, sum, and integrate beamforming (burst-wise averaging) correlated linearly with MRI-assessed tissue damage volumes (Fig.5), and predicted the damage volume centroid locations (error = 1.9 ± 0.7 mm).Discussion
3D ultraharmonic imaging can be used to calibrate exposure levels for microbubble-mediated FUS brain therapy, and can predict the MRI-inferred tissue damage volume distributions resulting from high target level sonications. The spatial information provided by PCI is expected to improve acoustic emissions-based control schemes for cavitation-based FUS therapies, both in the brain and in other parts of the body.Acknowledgements
The authors thank S. Rideout-Gros, V. Chan, and M. Harvey for help with animal care, and S. Gunaseelan, W. Li, A. Rajkumar, J. Zhou, N. Mehdizadeh, T. Jakaza, and K. Sinha for technical support. Financial support for this work was provided by the Weston Family Foundation, Beamer and Connor Foundations, National Institutes of Biomedical Imaging and Bioengineering of the National Institutes of Health (R01 EB003268), the Canadian Institutes of Health Research (FRN 119312), and the Temerty Chair in Focused Ultrasound Research at Sunnybrook Health Sciences Centre.References
1. Hynynen K et al. Noninvasive MR imaging-guided focal opening of the blood-brain barrier in rabbits. Radiology. 2001;220(3):640-6.
2. McDannold NJ et al. Microbubble contrast agent with focused ultrasound to create brain lesions at low power levels: MR imaging and histologic study in rabbits. Radiology. 2006;241(1):95-106.
3. O’Reilly MA et al. Three-dimensional transcranial ultrasound imaging of microbubble clouds using a sparse hemispherical array. IEEE Trans. Biomed. Eng. 2014;61(4):1285-94.
4. Crake C et al. A dual-mode hemispherical sparse array for 3D passive acoustic mapping and skull localization within a clinical MRI guided focused ultrasound device. Phys. Med. Biol. 2018;63(6):065008.
5. Jones RM et al. Three-dimensional transcranial microbubble imaging for guiding volumetric ultrasound-mediated blood-brain barrier opening. Theranostics. 2018;8(11):2909-26.
6. Jones RM et al. Ultrafast three-dimensional microbubble imaging in vivo predicts tissue damage volume distributions during nonthermal brain ablation. Theranostics. 2020;10(16):7211-30.
7. Ellens NPK et al. A novel, flat, electronically-steered phased array transducer for tissue ablation: preliminary results. Phys. Med. Biol. 2015;60(6):2195-215.
8. Dadgar M et al. High-pressure low-frequency lateral mode phased-array transducer system for the treatment of deep vein thrombosis: an in vitro study. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2022;69(3):1088-99.
Figures