1063
In vivo performance of a wearable coil vest for 3 T breast MRI (BraCoil)1High Field MR Center, Medical University of Vienna, Vienna, Austria, 2Laboratoire d’Imagerie Biomédicale Multimodale Paris Saclay (BioMaps), CEA, CNRS, Inserm, Université Paris-Saclay, Paris-Saclay, France, 3IADI, Inserm, Université de Lorraine, Nancy, France
Synopsis
Keywords: New Devices, RF Arrays & Systems
Recently, we introduced the “BraCoil”, a wearable coil for prone and supine breast imaging designed to overcome several shortcomings in clinical breast MRI. Here, we present in vivo performance tests on 12 volunteers with different breast volumes ranging from 495 mL (bra size 70A) to 3020 mL (90D). SNR gain up to a factor of three over a commercial breast coil was found. Acceleration factors up to 6x4 can be used with reasonable g-factors. High-quality in vivo images with different contrasts are presented.Introduction
Breast cancer is the most common malignant tumor in women worldwide1. Early diagnosis is important to reduce mortality. Digital X-ray mammography is the standard screening modality, simple and cost-effective2, but has low sensitivity in dense breasts3. In addition, the required breast compression causes discomfort or even pain, and X-ray based screenings are often not recommended below the age of 45 years4 due to the risks inherent to ionizing radiation.All these limitations can be alleviated by breast MRI, which yields excellent soft tissue contrast, and has the highest sensitivity for breast cancer detection5, regardless of breast density6. Typically, breast MRI is performed with the subject lying in prone position with exposed breasts hanging into cup-shaped RF coils. These rigid coils are designed for women with large breasts, yielding limited signal-to-noise ratio (SNR) for smaller breasts. Besides the uncomfortable position, image fusion for diagnosis and treatment such as US-guided biopsy or surgery, typically performed in supine position, is complicated.
To overcome these shortcomings, we designed a wearable breast coil (“BraCoil”)7. In this work, the coil’s in vivo performance is evaluated on volunteers: an SNR comparison to a commercial breast coil, the BraCoil’s parallel imaging performance, and images with clinically relevant contrasts are presented.
Methods
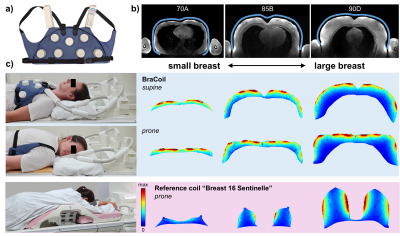
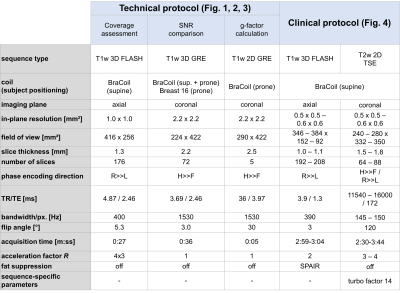
The BraCoil (Fig. 1a) is a flexible 28-channel receive-only array made from coaxial coils arranged in 4-element modules8. Its performance was tested in 12 healthy female volunteers with bra sizes ranging from 70A to 90D (European standard, EN13402), age 31±5 years and mean body mass index 23±4 kg/m² in supine and prone position on a 3T MR scanner (Prisma Fit, Siemens Healthineers, Erlangen, Germany) after approval of the local ethics committee (Medical University of Vienna, Vienna, Austria, No. 2137/2021) and written informed consent.Axial T1-weighted 2D gradient echo (GRE) images with intentionally low contrast between glandular and fat tissue were acquired to show the sensitive area of the BraCoil. SNR maps were calculated from 3D GRE imaging data and noise-only scans9, which were acquired with the BraCoil and a clinical breast coil (“Breast 16 Sentinelle”, Siemens Healthineers, Erlangen, Germany) as a reference. For both coils, the SNR was evaluated in a region of interest covering the entire breast volume which was manually segmented using 3D slicer10. Additionally, g-factor maps with a simulated acceleration in left-right (LR) from 2-8 and head-foot (HF) from 2-6 were calculated using the multiple pseudo replica method9,11. T1-weighted images with fat suppression and T2-weighted turbo spin echo (TSE) images were acquired. The volunteers were instructed to perform abdominal breathing to minimize chest motion. Pulse sequence parameters are summarized in Tab. 1.
Results and Discussion
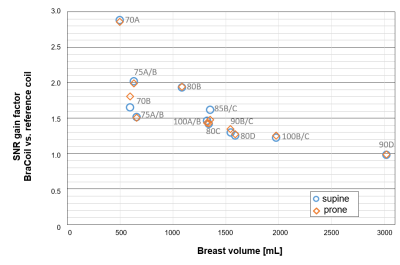
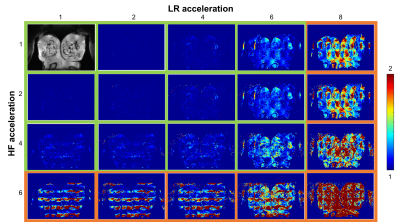
Fig. 1b illustrates the BraCoil’s sensitive area covering all investigated breast sizes. SNR maps in Fig. 1c show that with the reference coil high SNR can only be achieved in large breasts, whereas the BraCoil’s sensitivity is high for all different subjects. The SNR gain of the BraCoil over the reference coil is plotted against the breast volume of each subject in Fig. 2. The gain ranges from a factor of three for small breasts to equal SNR for large breasts. Similar results were obtained for the BraCoil used in the supine and the prone position.In Fig. 3 the maximum acceleration factor for parallel imaging with g-factors <2 was determined as 6x4=24.
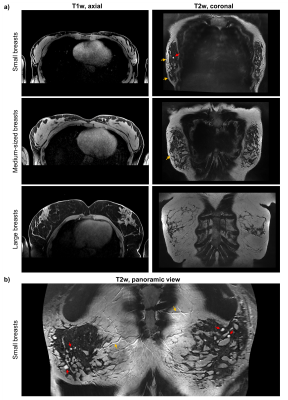
T1- and T2-weighted images in Fig. 4a acquired with resolutions higher than in typical clinical breast imaging protocols show fine anatomical details and are of diagnostic quality. However, for supine breast MRI, and in particular for smaller breasts, the visualization in Cartesian axial or coronal view is rather inefficient due to the curved geometry of the breasts. Therefore, we propose a novel panoramic view similar to dental panoramic X-ray. In Fig. 4b such a panoramic view, created from the same data as in Fig. 4a (top), outlines improved image reading. The proposed method is described in detail in a companion abstract.
Image acquisition in supine position naturally entails motion of the subjects’ chest, which was of little concern for our healthy volunteer experiments, since they complied well with the instructions to perform shallow abdominal breathing. However, this issue needs to be addressed in patients that are in a more stressful situation. Therefore, the aim of ongoing work is to equip the coil with motion sensors12 and to implement online retrospective motion correction techniques13.
Conclusion
The wearable coil (“BraCoil”) was successfully tested in 12 healthy volunteers in supine and prone position. The coil was shown to be suitable for imaging small and large breasts alike, with an upper limit above 90D yet to be determined. Compared to a commercial breast coil, an SNR gain up to a factor of three was obtained. The high possible parallel imaging acceleration with the BraCoil is enabled by its high channel count (both in LR and HF direction). High-quality in vivo images with different contrasts were presented.We believe that the BraCoil could significantly improve patient comfort and future breast cancer care. It enables supine breast MRI which conserves a natural breast shape, and thereby facilitates the translation of MR imaging findings to targeted ultrasound examinations, biopsy, and surgery.
Acknowledgements
This work was funded by the joint Austrian/French grant “BRACOIL“ (Austrian Science Fund FWF Nr. I-3618/Agence Nationale de Recherche ANR-17-CE19-0022), the Austrian Society of Senology, Anniversary Fund of the Oesterreichische Nationalbank (Austrian Central Bank, project nr. 17980 “FlexShim“).References
1. Forouzanfar, M. H. et al. Breast and cervical cancer in 187 countries between 1980 and 2010: a systematic analysis. The Lancet 378, 1461–1484 (2011).
2. Feig, S. Cost-Effectiveness of Mammography, MRI, and Ultrasonography for Breast Cancer Screening. Radiologic Clinics of North America 48 879–891 (2010).
3. Lewin, J. M., Isaacs, P. K., Vance, V. & Larke, F. J. Dual-energy contrast-enhanced digital subtraction mammography: feasibility. Radiology 229, 261–268 (2003).
4. Peintinger, F. National Breast Screening Programs across Europe. Breast Care 14, 354–358 (2019).
5. Pötsch, N., Vatteroni, G., Clauser, P., Helbich, T. H. & Baltzer, P. A. T. Contrast-enhanced Mammography versus Contrast-enhanced Breast MRI: A Systematic Review and Meta-Analysis. Radiology 305, 94–103 (2022).
6. Mann, R. M. et al. Breast cancer screening in women with extremely dense breasts recommendations of the European Society of Breast Imaging (EUSOBI). Eur. Radiol. 32, 4036–4045 (2022).
7. Obermann, M. et al. BraCoil – a wearable one-size-fits-all breast coil for 3 T MR mammography. in Proc. Intl. Soc. Mag. Reson. Med. 30 (2022) p. 188.
8. Obermann, M., Roat, S. & Laistler, E. Coaxial coil modules as building blocks of individually arranged receive-only coil arrays. in Proc. Intl. Soc. Mag. Reson. Med. 29 (2021) p. 1610.
9. Robson, P. M. et al. Comprehensive quantification of signal-to-noise ratio and g-factor for image-based and k-space-based parallel imaging reconstructions. Magn. Reson. Med. 60, 895–907 (2008).
10. Fedorov, A. et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn. Reson. Imaging 30, 1323–1341 (2012).
11. Montin, E. & Lattanzi, R. Seeking a widely adoptable practical standard to estimate signal-to-noise ratio in magnetic resonance imaging for multiple-coil reconstructions. J. Magn. Reson. Imaging 54, 1952–1964 (2021).
12. Chen, B. et al. Design and Validation of a Novel MR-Compatible Sensor for Respiratory Motion Modeling and Correction. IEEE Trans. Biomed. Eng. 64, 123–133 (2017).
13. Odille, F., Vuissoz, P.-A., Marie, P.-Y. & Felblinger, J. Generalized reconstruction by inversion of coupled systems (GRICS) applied to free-breathing MRI. Magn. Reson. Med. 60, 146–157 (2008).
Figures