1062
3 Tesla 31P/1H Calf Muscle Coil for 1H and 31P MRI / MRS integrated with NIRS
Bei Zhang1, Daniel Lowrance1, David Zaha1, Manoj Kumar Sarma1, Michael Douglas Nelson2, and Anke Henning1
1Advanced Imaging Research Center, UT Southwestern Medical Center, Dallas, TX, United States, 2The University of Texas at Arlington, Arlington, TX, United States
1Advanced Imaging Research Center, UT Southwestern Medical Center, Dallas, TX, United States, 2The University of Texas at Arlington, Arlington, TX, United States
Synopsis
Keywords: Non-Array RF Coils, Antennas & Waveguides, Skeletal, 31P/1H Dual-tuned, NIRS
In this work, we present a 3T 31P/1H calf coil with two interleaved and co-centered birdcages in one layer to provide homogenous transmit fields and good SNR for both 31P and 1H nuclei. In addition, the coil design allows for integration of a Near-infrared spectroscopy (NIRS) probe for simultaneous NIRS and MRI readouts during exercise. Simulation and phantom experimental results show that the coil provides homogeneous transmit and receive fields. In vivo experiment show that the coil provide good SNR for both 31P MRS and 1H MRI and MRSINTRODUCTION
31P MRS directly estimates the oxidative adenosine triphosphate (ATP) synthesis rate in skeletal muscle during exercise and recovery (1-3), while 1H MRI and MRS can provide complimentary information regarding oxygen delivery (i.e. skeletal muscle perfusion), oxygen utilization (i.e. venous oxygen saturation), and differentiates intramyocellular from extramyocellular lipids. Therefore, it is desirable to have a 31P/1H calf coil designed for providing both good 31P and 1H transmit and receive performance. An interleaved layout of two phased arrays in one layer has been found to provide optimal transmit and receive performances for both nuclei at ultra-high field (4,5). However, at 3T and lower fields, the strong coupling between coil elements makes phased array designs difficult. Birdcage is an excellent alternative option for 31P/1H calf coil design at 3T. In this case, birdcage provides homogenous transmit and receive fields for both nuclei, which are essential for image-based evaluations of perfusion or fat distribution as well as 1H and 31P MRS. In this work, we thus present a 31P/1H calf coil with two interleaved birdcages in one layer to provide homogenous transmit fields and good SNR for both 31P and 1H. In addition, the coil design allows for integration of a NIRS probe for simultaneous NIRS and MRI readouts during exercise.METHOD
Full-wave simulation: Microwave Studio in CST (Dassault Systèmes) is used to model an interleaved and co-centered 31P high-pass birdcage and 1H low-pass birdcage, loaded with a 2mm isotropic Duke leg (Figure 1a) (6). The 31P birdcage’s diameter is 210mm, length 200mm, and copper width 15mm, while the 1H birdcage’s diameter is 212mm, length 150mm, and copper width 8mm. 50ohm ports were placed at each gap on the birdcages, and co-simulation tool in CST was used for tuning and matching with two quadrature-driven ports symmetrically located 45° away from the bottom of the leg. B1+ maps and SNR were calculated for 31P and 1H. Coil prototyping: Figure 1b shows the CAD model of the coil housing, including a coil holder, an interface box, a NIRS probe holder, and a coil support. The birdcages are mounted inside the coil holder and have the same lengths and copper widths as specified for the simulation. A 0.78mm-thick FR4 block was used to separate the two birdcages at each conductive-overlap area (Figure 1c). After matching , each port was connected to a shielded 31P/1H dual-tuned cable trap, and then connected to a TR-switch integrated on a preamplifier board. A 31P/1H quadrature hybrid was used to drive the two ports of the 31P/1H birdcage (Figure 1d). Figure 1e shows setup of the NIRS probe when working with the coil. The NIRS device is held in a NIRS insert (Figure 1f). Phantom experiments: B1+ maps and SNR in a methyl phosphate phantom (cylinder-shaped, 2 g/L) were measured to characterize the spatial coverage and performance of the 31P and 1H birdcages. For 31P, B1+ map was calculated with a 2D CSI sequence using the double angle method; and the same 2D CSI sequence with/without RF excitation was used to calculate SNR voxel-by-voxel by dividing the peak absolute signal by the standard deviation of noise. For 1H, SNR maps were reconstructed from two GRE sequences with/without RF excitation, and a Turbo Field Echo (TFE) sequence was used to acquire the B1+ maps (7). In vivo Experiments: Human subject studies were performed on a Siemens Prisma 3T MRI system (Siemens Healthcare) with institutional review board approval. In vivo 1H T1w images were acquired with a 3D MPRAGE sequence. An in vivo 1H MR spectrum was acquired with PRESS sequence; and in vivo 31P spectra were acquired with the same 2D CSI sequence as described above.RESULT AND DISCUSSION
The unloaded to loaded ratio is 8.32 for the 31P birdcage, and 10.5 for the 1H birdcage. Table 1 lists the capacitance of each tuning capacitor in simulation, prototype, and theoretically calculated for a single birdcage (8). These values are very similar, demonstrating that the two birdcages do not strongly couple. The matching of one birdcage also does not change much with/without the other birdcage being present and resonant, as observed during assembly and workbench testing. Therefore, no LCC trap was implemented. Figure 2 shows the simulated SNR and B1+ in the Duke leg and the experimental SNR and B1+ in the phantom of the 1H and 31P birdcages. In both simulation and experiment, SNR and B1+ are slightly stronger in area close to the two ports for both 1H and 31P, but homogenous in the center area. The 1H MPRAGE images in Figure 3 show anatomical details of the calf, and a representative 1H MR spectrum shows intramyocellular (IMCL) and extramyocellular lipids (EMCL). Figure 4 shows in vivo 31P MRSI data of the calf muscle in the transversal plane, and a representative 31P MR spectrum in one of the voxels. The 31P MRSI data show that the peak of 31P MR spectra in voxels in the center area are similar, indicating homogeneous 31P signal intensity.CONCLUSION
The 3T 31P/1H calf coil with two interleaved birdcages described herein provides homogeneous transmit and receive fields and good SNR for both 31P MRS and 1H MRI and MRS.Acknowledgements
This work was funded by Cancer Prevention and Research Institute of Texas (CPRIT) RR180056 and was performed under the rubric of the Advanced Imaging Research Center, UT Southwestern Medical Center.References
- Kemp GJ, Taylor DJ, Thompson CH, Hands LJ, Rajagopalan B, Styles P, Radda GK. Quantitative analysis by 31P magnetic resonance spectroscopy of abnormal mitochondrial oxidation in skeletal muscle during recovery from exercise. NMR Biomed 1993;6(5):302-310.
- Sanderson AL, Kemp GJ, Thompson CH, Radda GK. Increased oxidative and delayed glycogenolytic ATP synthesis in exercising skeletal muscle of obese (insulin-resistant) Zucker rats. Clin Sci (Lond) 1996;91(6):691-702.
- Wu F, Jeneson JA, Beard DA. Oxidative ATP synthesis in skeletal muscle is controlled by substrate feedback. Am J Physiol Cell Physiol 2007;292(1):C115-124.
- Avdievich NI, Ruhm L, Dorst J, Scheffler K, Korzowski A, Henning A. Double-tuned (31) P/(1) H human head array with high performance at both frequencies for spectroscopic imaging at 9.4T. Magn Reson Med 2020;84(2):1076-1089.
- Wang B, Zhang B, Yu Z, Ianniello C, Lakshmanan K, Paska J, Madelin G, Cloos M, Brown R. A radially interleaved sodium and proton coil array for brain MRI at 7 T. NMR Biomed 2021;34(12):e4608.
- Christ A, Kainz W, Hahn EG, Honegger K, Zefferer M, Neufeld E, Rascher W, Janka R, Bautz W, Chen J, Kiefer B, Schmitt P, Hollenbach HP, Shen J, Oberle M, Szczerba D, Kam A, Guag JW, Kuster N. The Virtual Family--development of surface-based anatomical models of two adults and two children for dosimetric simulations. Phys Med Biol 2010;55(2):N23-38.
- Chung S, Kim D, Breton E, Axel L. Rapid B1+ mapping using a preconditioning RF pulse with TurboFLASH readout. Magn Reson Med 2010;64(2):439-446.
- BirdcageBuilder Mobile. https://cai2rnet/resources/birdcagebuilder-mobile/.
Figures
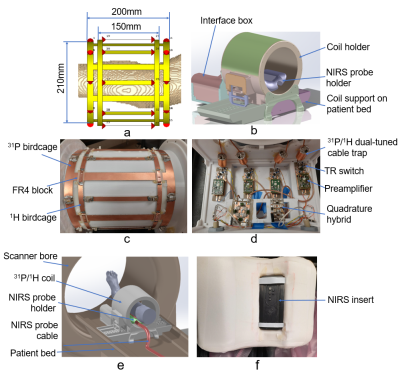
Figure 1. a) Modeling of the 31P and 1H birdcages, loaded with 2mm isotropic Duke leg (truncated for viewing), the 31P
birdcage's diameter is 210mm and length 200mm, while 1H birdcage’s
diameter 212mm and length 150mm; b) CAD of the coil housing, including coil holder, interface box, NIRS probe
holder and coil support; c) 31P and 1H
birdcages, 0.078mm-thick FR4 bocks at conductive overlap areas; d) coil interface including 31P/1H
dual-tuned cable traps, TR switches, preamplifiers and quadrature hybrids; e) NIRS probe setup with the coil; f) NIRS device positioning inside the NIRS holder
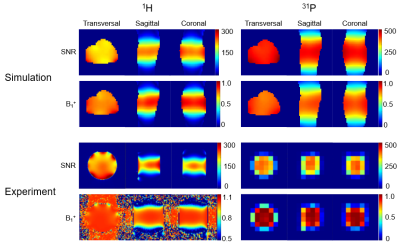
Figure 2 Simulated SNR and B1+
in the Duke leg, and experimental ones in the methyl phosphate phantom. 1H SNR maps were reconstructed from 2 GRE sequences (FOV = 300×300mm, matrix
= 160×160,
TR = 200 ms, TE = 3.0 ms, FA = 25°) with/without RF excitation, and FA maps were acquired from a 2D TFE (FOV
= 300×300mm,
matrix
= 160×160, TR
= 2000 ms, TE = 1.8 ms). 31P SNR maps were calculated from 2D CSI with (FA=60°) and without RF
excitation, and B1+ maps were calculated from two 2D CSI sequences (FA=30°/60°, slice thickness = 25mm,
matrix = 12x12, FOV = 300 x 300mm, TR = 3000ms, ave. = 2, BW=4000 Hz/pixel).
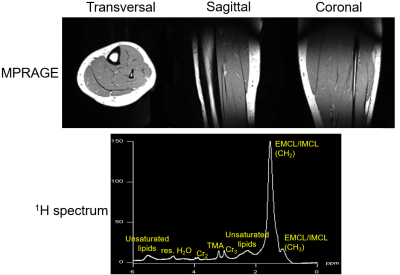
Figure 3. 1H MPRAGE images (resolution=1.0×1.0×1.0mm,
FOV=256×256×256mm,
FA=8°, TR=1800 ms, TE=2.26 ms) of the human calf
in transversal, sagittal and coronal planes respectively (top) and 1H
MR spectrum acquired with a PRESS sequence (resolution = 25mm×25mm×25mm, TR=2000ms, TE=30ms, ave.=128, and BW=2000 Hz/pixel) showing IMCL and EMCL (bottom)
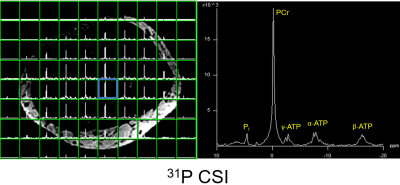
Figure 4. 31P MRSI data acquired from
human calf muscle in the transversal plane (left) and a 31P MR
spectrum from the voxel marked by the blue square, acquired with a 2D CSI sequence
(slice thickness = 25 mm,
matrix size = 12x12, FOV = 240 x 240mm, TR = 2000ms, TE = 2.3 ms, averages = 1, and BW=4000 Hz/pixel)

Table 1: Tuning capacitor in simulation,
prototype, and theoretically calculated for single birdcage in BirdcageBuilder
DOI: https://doi.org/10.58530/2023/1062