1060
Performance Evaluation of a 128-Channel head-only Receiver array at 7 Tesla1A.A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Charlestown, MA, United States, 2High Field MR Center, Center for Medical Physics and Biomedical Engineering, Medical University Vienna, Vienna, Austria, 3BARNLabs, Muenzkirchen, Austria, 4Harvard Medical School, Boston, MA, United States, 5Harvard-MIT Division of Health Sciences and Technology, Cambridge, MA, United States, 6Siemens Medical Solutions USA Inc., Malvern, PA, United States, 7Department of Life Science Engineering, Institute of Medical Physics and Radiation Protection, Mittelhessen University of Applied Sciences, Gießen, Germany, 8Advanced MRI Technologies, Sebastopol, CA, United States, 9Helen Wills Neusoscience Institute, University of California, Berkeley, CA, United States
Synopsis
Keywords: RF Arrays & Systems, RF Arrays & Systems
The performance of a 128-channel Rx-only 7T brain array was evaluated using simulations and measurements. SNR and g-factor maps show a significant performance increase for highly accelerated imaging in cortical areas from a combination of improved peripheral unaccelerated SNR and g-factor. Measured SNR in cortical areas increased by 42% from 32- to 128-ch and 18% from 64- to 128-ch. The 1/g-factor maps show an improved mean and a tighter distribution, with both effects becoming more pronounced at higher accelerations. At 6x2-fold the 128-channel array has 17.9% g-factor benefit over the 64-ch, and a 48.2% benefit over the 32-ch array.Introduction
Along with the increased field strength, high-channel count brain arrays can increase the Signal-to-Noise (SNR) ratio, particularly at the brain periphery where this added SNR benefits high-resolution cortical fMRI [1,2]. Furthermore, increasing the Rx channel count also increased the acceleration factor (R) that can be used for parallel imaging [3,4,5,6]. For functional brain imaging, increasing R can be used either to reduce image distortion or to increase the achievable resolution at a given echo time (TE) [7,8]. While 32-channel brain arrays have become standard at 7T [9], the potential SNR and parallel imaging benefits of 64- and 96-channel 7T arrays have recently been investigated [10,11,12]. In this work we present the first in-vivo data acquired with a 128-channel brain array at 7T. Building on a previous simulation study [13], we show the advantage of the 128-ch array over a commercial 32-ch and a research-only 64-ch head array for 7T acquisitions in measured SNR and g-factor maps.Methods
The construction of the 128ch array has been previously described [13]. Simulations compared the performance of the 128-channel array and its loop layout, to a home-built 32-channel [14], and 64-channel head array [10]. SNR simulations for the three arrays used the “MARIE” fast electromagnetic full-wave solver [15] and a meshed homogeneous head-neck numerical phantom (average brain: ε=52, σ=0.55 S/m) with (1x1x1) mm3 [16]. Coil copper losses were estimated from bench Q-measurements of the three loop sizes of the three different arrays used.Measurements compared a commercial 32-channel [9], and a home-built 64-channel head array [10] on a conventional Siemens Terra 7T system (Siemens Healthineers, Erlangen, Germany) with a whole-body SC72 gradient coil (Gmax = 70 mT/m; Slew Rate = 200 T/m/s) to the measurements with the 128-channel head array [13], performed on a modified Siemens Terra 7T scanner (NexGen 7T at Berkeley) with a high-performance head-only gradient coil (Gmax = 200 mT/m; Slew Rate = 900 T/m/s) and a 128 channel receiver system [17]. All three RF arrays used a quadrature birdcage coil for transmit. Receive SNR measurements used a whole-brain 2D proton-density weighted gradient-echo sequence with a nominal flip-angle of 90° to limit the impact of B1+ inhomogeneities on the signal intensity [TR/TE/flip angle (FA) = 5s/3.82ms/90°, slice = 2 mm, matrix = 256x88, FOV = 256x176 mm2, readout bandwidth (BW) = 335 Hz/pixel, TA=7:22 min]. Noise covariance information was acquired using the same pulse sequence, but without RF excitation. Following the method of Kellmann et al., SNR maps used the noise covariance-weighted optimal coil combination of the individual channel images, where the weights use coil sensitivity maps and noise-covariance information [16, 18]. Birdcage coil flip angle (FA) maps were acquired using a pre-conditioning saturation pulse with a turbo-flash readout [19] [TR/TE/FA = 5s/2.02ms/90°, slice = 1.5 mm, matrix = 256x88, FOV = 256x128 mm2, BW = 335 Hz/pixel, Turbo factor = 128]. The SNR maps were then normalized by dividing them by sin(FA) in order to isolate the receive sensitivity in the SNR maps.
G-factor maps were computed from the same 2D proton density-weighted GRE data as the SNR maps. ESPIRiT [20] was used to estimate the coil receive sensitivity maps from the brain image data. Retained g-factor maps (1/g) were computed in axial, sagittal and coronal direction for several in-plane and SMS accelerations with CAIPIRINHA (FOV shifting). Mean and maximum g values were determined after the application of a brain mask and a smoothing filter on the region of interest.
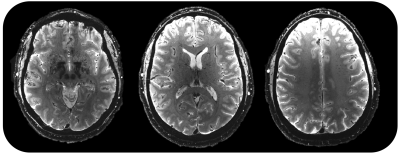
Finally, whole brain T2*-weighted high resolution structural images were acquired at 300 µm in-plane resolution using a 2D multi-slice gradient-recalled echo (GRE) sequence using the 128-channel array.
Results
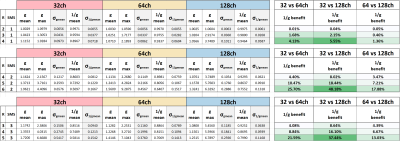
Figure 1 shows good agreement between simulated and measured SNR maps for unaccelerated imaging. The measured SNR at the brain center is comparable for all three arrays, whereas the cortical areas show an average SNR increase of 42% from 32- to 128-channels and 18% increase from 64- to 128-receive channels.Inverse g-factor maps for the three measured arrays are shown in Figure 2. In 6x2-fold accelerated multi-slice acquisitions, the 128-channel array provides a reduction of 33% and 14.6% in mean and max g-factor compared to the 32-channel array. The reduction is 15.4% and 31.9% compared to the 64-channel array.
Histogram plots of retained SNR (1/g) for all three arrays are shown in Figure 4 as violin plots. The 128ch array consistently shows narrower histograms that are clustered closer to unity. For example, at 6x2-fold acceleration the 128-channel array has 17.9% benefit over the 64-ch, and a 48.2% benefit over the 32-ch array in g-value distribution. Table 1 tabulates the acceleration results.
Figure 4 shows images from a 2D multi-slice gradient echo acquisition with the 128-channel coil and the high-performance gradient coil on the modified 7T system.
Discussion and Conclusion
We demonstrate significant gains in accelerated imaging performance using a 128-ch Rx brain array at 7T as compared to 32-ch and 64-ch arrays. Commensurate gains in unaccelerated cortical SNR are seen in both simulations and measurements, and gains in retained SNR (1/g) are achieved over the whole brain. The 128-ch Rx array thus works synergistically with the high-performance gradient coil to support sub-millimeter functional imaging in the human cortex.Acknowledgements
Research reported in this publication was supported by the NIH BRAIN Initiative, the National Institute of Biomedical Imaging and Bioengineering, under award number U01EB025162. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.References
[1] Wiggins GC., et al., 96-channel receive-only head coil for 3 Tesla: Design optimization and evaluation. MRM, 2009, 62(3): 754–762.
[2] Ugurbil K, et al., Brain imaging with improved acceleration and SNR at 7 Tesla obtained with 64-channel receive array. MRM, 2019, 82(1): 495–509.
[3] Sodickson DK., Simultaneous acquisition of spatial harmonics (SMASH): Fast imaging with radiofrequency coil arrays. MRM, 1997, 38: 591–603.
[4] Griswold MA., et al., Generalized autocalibrating partially parallel acquisitions (GRAPPA). MRM, 2002, 47(6): 1202–1210.
[5] Pruessmann KP., et al., SENSE: Sensitivity encoding for fast MRI. MRM, 1999, 42(5): 952–962.
[6] Wiesinger F., et al., Potential and feasibility of parallel MRI at high field. NMR in biomedicine, 2006, 19: 368–378.
[7] Triantafyllou C., et al., Comparison of physiological noise at 1.5 T, 3 T and 7 T and optimization of fMRI acquisition parameters. NeuroImage, 2005, 26(1): 243–250.
[8] Triantafyllou C., et al., Physiological noise and signal-to-noise ratio in fMRI with multi-channel array coils. NeuroImage, 2011, 55(2): 597–606.
[9] Ledden PJ., et al., A 32-channel receive-only SENSE Array for brain imaging at 7T. In: Proceedings of the 15th ISMRM Annual Meeting and Exhibition, Berlin, Germany, 2007, #0242.
[10] Mareyam A., et al., A 64-channel 7T array coil for accelerated brain MRI. In: Proceedings of the ISMRM Annual Meeting and Exhibition, Online, 2020, #0242.
[11] Gunamony S., et al., A 16-channel transmit 96-channel receive head coil for NexGen 7T scanner. In: Proceedings of the ISMRM Annual Meeting and Exhibition, Online, 2021, #0182.
[12] May MW., et al., A patient-friendly 16-channel transmit/64-channel receive coil array for combined head–neck MRI at 7 Tesla. MRM, 2022, 88: 1419–1433.
[13] Gruber B., et al., A 128-channel head coil array for Cortical Imaging at 7 Tesla. In: Proceedings of the ISMRM Annual Meeting and Exhibition, Online, 2021, #0176.
[14] Keil B., et al. Design Optimization of a 32-channel Head Coil at 7T. In: Proceedings of the ISMRM Annual Meeting and Exhibition, Stockholm, 2010, #1493.
[15] Villena JF., et al., Fast Electromagnetic Analysis of MRI Transmit RF Coils Based on Accelerated Integral Equation Methods. IEEE Trans. Biomed. Engine., 2016, 63: 2250–2261.
[16] Roemer PB., et al., The NMR phased array. MRM, 1990, 16(2): 192–225.
[17] Feinberg DA., et al., Design and Development of a Next-Generation 7T human brain scanner with high-performance gradient coil and dense RF arrays. In: Proceedings of the ISMRM Annual Meeting and Exhibition, Online, 2021, #0562.
[18] Kellman P., et al., Image reconstruction in SNR units: A general method for SNR measurement. MRM, 2005, 54(6): 1439–1447.
[19] Chung S., et al. Rapid B1+ Mapping using a Pre-Conditioning RF Pulse with TurboFLASH readout. MRM, 2010, 64(2): 439–446.
[20] Uecker M., et al., ESPIRiT-an eigenvalue approach to autocalibrating parallel MRI: Where SENSE meets GRAPPA. MRM, 2014, 71: 990–1001.
Figures