1051
Tissue orientation effects on T2 relaxation in fresh and fixed spinal cord white matter1Physics and Astronomy, University of British Columbia, Vancouver, BC, Canada, 2UBC MRI Research Center, Vancouver, BC, Canada, 3Pediatrics, University of British Columbia, Vancouver, BC, Canada, 4UBC MRI Research Centre, Vancouver, BC, Canada, 5Radiology and Urologic Sciences, University of British Columbia, Vancouver, BC, Canada, 6Neuroradiology, Medical University of Innsbruck, Innsbruck, Austria, 7Djavad Mowafaghian Centre for Brain Health, University of British Columbia, Vancouver, BC, Canada
Synopsis
Keywords: Microstructure, White Matter, Relaxometry
In this study, we investigated the orientation dependence of T2 in fresh and fixed spinal cord white matter (WM). Scans from three pig spinal cord tissues were acquired at 7T at 6 different orientations with respect to the main magnetic field. We found a considerable orientation dependence in the short fraction relaxation rate R2 (=1/T2) in fresh WM as opposed to a weak orientation effect in fixed WM. To our knowledge, this is the first direct comparison of orientation dependence in a WM tissue sample in the fresh and fixed state. Orientation dependence was not observed in long fraction R2.Introduction
Understanding the central nervous system at the microstructural level is one of the key endeavors of neuroscience. Important progress has been made in the past decades thanks to major advances in MRI of the brain and spinal cord. Despite these achievements, we still lack a complete picture of how tissue architecture influences the fundamental contrast mechanisms in MRI. In recent years, it has been shown that the T2 relaxation time of the MRI signal in ordered tissue depends on the nerve fiber orientation relative to the main magnetic field1-3. Previous work found a considerable dependence in brain white matter in vivo1, in contrast to a weak orientation effect in fixed white matter tissue4,5. This project aims to supplement our understanding of the reduction of orientation dependency in fixed tissue by measuring T2 relaxation in white matter tissue before and after fixation. This study will serve as validation work for future orientation dependency experiments.Methods
White matter tissue was obtained via ongoing collaborations with the International Collaboration on Repair Discoveries (ICORD) for animal models. After a pilot measurement, three uninjured samples of fresh pig spinal cord tissue were imaged using a multi-echo spin echo sequence at six different orientations with respect to the main magnetic field; 64 echoes, TR = 1720 ms and TE = 6.75 ms. The angles ranged from 0° (parallel to B0) to 90° (perpendicular to B0), including the magic angle 54.7°. The orientation was manipulated using a sample holder that permits sample rotation inside the MRI scanner.After an initial scan of fresh tissue, the tissue underwent fixation and remained immersed in formaldehyde for 20 days prior to second scanning. All samples underwent the same imaging protocol and all experiments were performed on a 7T preclinical MRI scanner (Bruker Biospin, Germany).
Data was denoised using a non-local means algorithm6, followed by computation of the T2 distributions using DECAES7; $$$\chi^2$$$ regularization and 101 logarithmically spaced T2 components were used. Three regions of interest (ROI) – the dorsal columns (DC), ventral columns (VC) and lateral columns (LC) – were manually outlined for each orientation. White matter masks were made using 3DSlicer8.
Results
Figure 1 showcases the maps of the T2 relaxation time for the fresh cords. The relaxation rate R2 (=1/T2) as a function of angle is presented in Figure 2.Short fraction R2: fresh tissue presented a visible orientation dependence in all ROIs. Values increased with angle from 80 Hz at low angles (close to 0°) to 120 Hz at high angles (close to 90°). Short fraction R2 values for fixed tissue showed a mild increase of about 10 Hz with increasing angle across all ROIs.
Long fraction R2: no considerable orientation dependence was observed for either fresh or fixed tissue.
Discussion
The relaxation rate R2 of the short fraction, which is believed to correspond to myelin water, increased with increasing angle in fresh white matter. Myelin water is subject to field inhomogeneities due to the interactions between the main magnetic field and diamagnetic myelin. Orientation dependence of R2 has been previously seen and attributed to diffusion within such field inhomogeneities1,9. Given its cylindrical architecture10, the myelinated axon acts as an anisotropic structure and causes orientation dependency in the field inhomogeneities resulting in a change in R2.Fixed tissue orientation dependence follows a similar trend, but is strongly attenuated relative to fresh tissue. Short fraction R2 mildly increased with increasing angle in fixed white matter. Formalin reduces the diffusivity, lowering the mobility of molecules, which might reduce the effect from the field inhomogeneities caused by myelin1.
Contrary to our expectations, the relaxation rate R2 of the long fraction, which is believed to correspond to intra/extra cellular water, presented no considerable orientation dependence in any ROI. Previous work has found a bigger orientation effect in short than in long fraction R21. However, long fraction R2 still exhibited a dependence on white matter fiber orientation. It is worth noting that previous studies were performed in the human brain and that the tissue architecture is known to differ in other mammals11. Other mammals presented unmyelinated axons in spinal cord white matter11, which might have given rise to a more isotropic environment in our samples. The intra/extra cellular water was then subjected to less field inhomogeneities given its increased distance from myelin and it would be less likely to see angular dependence.
Conclusion
Short fraction R2 presented a considerable orientation dependence in fresh white matter. This trend in relation to angle is in agreement with previous in vivo studies1. The reduced orientation dependence observed in fixed white matter is also in accordance to previous work4. To our knowledge, this is the first study investigating orientation dependence of the same sample in the fresh and fixed state.Orientation dependence was noticeably reduced in the long fraction R2. In the next months, we will be acquiring 3 more cords and performing statistical analysis.
Acknowledgements
This work was conducted on the traditional, ancestral, and unceded territories of Coast Salish Peoples, including the territories of the xwməθkwəy̓əm (Musqueam), Skwxwú7mesh (Squamish), Stó:lō and Səl̓ílwətaʔ/Selilwitulh (Tsleil- Waututh) Nations.References
1. Birkl C, Doucette J, Fan M, Hernández‐Torres E, Rauscher A. Myelin water imaging depends on white matter fiber orientation in the human brain. Magnetic Resonance in Medicine. 2020;85(4):2221-2231. doi:10.1002/mrm.28543
2. Hänninen N, Rautiainen J, Rieppo L, Saarakkala S, Nissi MJ. Orientation anisotropy of quantitative MRI relaxation parameters in ordered tissue. Sci Rep. 2017;7(1):9606. Published 2017 Aug 29. doi:10.1038/s41598-017-10053-2
3. Bartels LM, Doucette J, Birkl C, Zhang Y, Weber AM, Rauscher A. Orientation dependence of R2 relaxation in the newborn brain [published online ahead of print, 2022 Oct 19]. Neuroimage. 2022;119702. doi:10.1016/j.neuroimage.2022.119702
4. Oh S-H, Kim Y-B, Cho Z-H, Lee J. Origin of B0 orientation dependent R2* (=1/T2*) in white matter. NeuroImage. 2013;73:71-79. doi:10.1016/j.neuroimage.2013.01.051
5. Alderson H, and Does M. Orientation Dependence of Myelin Water Fraction as Measured by MET2. Abstract presented at: 31st Annual Meeting of the International Society for Magnetic Resonance in Medicine; May 07-12, 2022; London, England, UK.
6. Coupé P, Yger P, Prima S, Hellier P, Kervrann C, Barillot C. IEEE Transactions on Medical Imaging, 27(4):425–441, 2008
7. Doucette J, Kames C, Rauscher A. DECAES - DEcomposition and Component Analysis of Exponential Signals. Z Med Phys. 2020;30(4):271-278. doi:10.1016/j.zemedi.2020.04.001
8. Fedorov A, Beichel R, Kalpathy-Cramer J, et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn Reson Imaging. 2012;30(9):1323-1341. doi:10.1016/j.mri.2012.05.001
9. Doucette J, Wei L, Hernández-Torres E, et al. Rapid solution of the Bloch-Torrey equation in anisotropic tissue: Application to dynamic susceptibility contrast MRI of cerebral white matter. Neuroimage. 2019;185:198-207. doi:10.1016/j.neuroimage.2018.10.035
10. Wharton S, Bowtell R. Fiber orientation-dependent white matter contrast in gradient echo MRI. Proc. Natl. Acad. Sci. U.S.A., 109 (45) (2012), pp. 18559-18564. doi:10.1073/pnas.1211075109
11. Saliani A, Perraud B, Duval T, Stikov N, Rossignol S, Cohen-Adad J. Axon and myelin morphology in animal and human spinal cord. Frontiers in Neuroanatomy. 2017;11. doi:10.3389/fnana.2017.00129
Figures
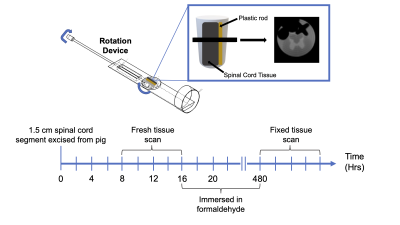
Figure 1: Experimental setup and timing.
Top half of the figure shows a schematic representation of the setup used to rotate the sample in the MRI and the first echo-image. A plastic rod (yellow) was used to prevent movement of the spinal cord tissue sample (dark gray).
Bottom half of the figure shows the timing of the scans.
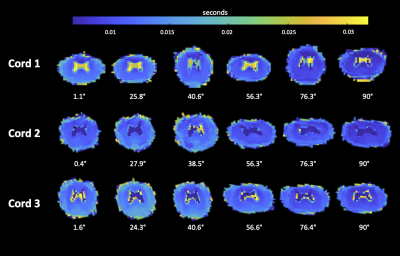
Figure 2: Voxel-by-voxel mapping of the short fraction T2 relaxation time for the fresh cords. The maps are the output of the multi-exponential analysis. Cords exhibit geometric distortion, possibly due to scanner imperfections.
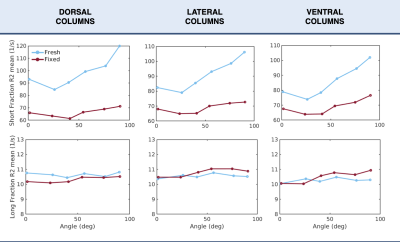
Figure 3: Averages of R2 as a function of orientation angle over all samples in three regions of interest. Fresh tissue is denoted in blue and fixed tissue in red. Data is presented using the relaxation rate R2 (=1/T2) given that it is most compatible with existing literature on orientation dependence.