1032
Deep Brain Stimulation of Nucleus Accumbens Alters Brain Functional Connectivity and Metabolism to Enhance Memory-Related Cognitive Function1Department of Biomedical Engineering, National Yang Ming Chiao Tung University, Taipei City, Taiwan, 2PhD Program in Medical Neuroscience, Taipei Medical University, Taipei City, Taiwan, 3Department of Neurology, Buddhist Tzu Chi Medical Foundation, Hualien County, Taiwan, 4Department of Neurology, Tzu Chi University, Hualien County, Taiwan
Synopsis
Keywords: Brain Connectivity, fMRI (resting state), deep brain stimulation, memory, cognition
Deep brain stimulation (DBS) has been a well-established treatment for cognitive dysfunction. However, cognitive dysfunctions were demonstrated to be associated with the metabolic syndrome. Nucleus accumbens (NAc) in the dopaminergic pathway is associated with glucose metabolism, considered to be a promising DBS targeted region to be investigated. Resting-state functional MRI, behavioral test, bioenergetic analysis and electron microscopy were applied in this study. We found increased functional connectivity, enhancement in cognitive behavior, increased energy metabolism and mitochondrial biomass after NAc-DBS.Introduction
Deep brain stimulation (DBS) is a useful technique for electrically stimulating specific brain regions for neuroplasticity change and circuit modification1,2. The ability of the brain to create neural networks for processing various events is known as neuroplasticity3. DBS has been observed to have an impact on mitochondrial biomass as well, including an increase in the size and quantity of mitochondria4. Electrical stimulation may promote cell growth and influence mitochondrial activity, according to a cellular study5,6. DBS has been shown to enhance cognitive function by modifying brain glucose levels7. DBS can therefore potentially be used to change the biomass and metabolism of the mitochondria. The nucleus accumbens (NAc) is a possible electrically stimulated area that could assist the connection between mitochondrial changes and dopaminergic pathway regulation based on the previous study8. Neuronal function in NAc was revealed to control brain circuits through mitochondrial structure in a rodent study9. NAc was also thought to play a significant role in cognitive function10. In this study, we assessed the effectiveness of NAc-DBS in the mice using resting-state functional MRI (rsfMRI) and behavioral task. The behavioral task of novel object recognition (NOR) was used to study recognition memory. The functional connectivity (FC) of dopaminergic pathway was investigated using the rsfMRI to clarify the NAc-DBS impact. Additionally, bioenergetic analysis of energy status was carried out in an effort to comprehend the metabolic change following NAc-DBS administration. After NAc-DBS, the mitochondrial biomass associated to energy metabolism was examined using electron microscopy (EM).Methods
Twenty male adult C57BL/6 mice were used in the study. Animals were divided into two groups: (1) sham controls (N = 10) and (2) NAc-DBS group (N = 10) to study the NAc-DBS effect in C57BL/6 mice. All mice had bilateral NAc implantation of MR-compatible neural probes11 (AP: + 1.1 mm, ML: 1.1 mm, DV: 4.0 mm). The NAc-DBS group received bilateral NAc-DBS treatments for 30 minutes every day for seven days at a frequency of 130 Hz, a pulse width of 60 μs, and an intensity of 200 μA. The long-term recognition memory was assessed using the NOR test, and the memory performance was determined by calculating the preference index (PI). Whole brain images were obtained using a 7 Tesla Bruker MRI scanner (Bruker Biospec 70/30 USR, Ettlingen, Germany) for pre- and post-DBS. The gradient-echo planar imaging sequence (TR / TE = 2,000 / 20 ms, FOV = 20 × 20 mm2, matrix size = 80 × 80, bandwidth = 200 kHz, 14 coronal slices, and thickness = 0.5 mm) was used to acquire the rsfMRI images. The Allen mouse brain atlas12 was used to determine the regions of interest (ROIs), which included the medial prefrontal cortex (mPFC), NAc, ventral hippocampus (vHIPP), and ventral tegmental area (VTA) (Figure 1A). The analysis of functional neuroimages (AFNI) program and the FMRIB Software Library v5.0 (FSL 5.0) were used to calculate FC, which was then normalized to the baseline (pre-DBS). The Seahorse XF24 analyzer (Agilent Technologies, Santa Clara, CA, USA) was used to identify the bioenergetic status of oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) using an assay protocol13. The energy status was represented by the OCR/ECAR ratio, which was determined. The energy state of the cells in the brain tissue was calculated using the OCR/ECAR ratio. By using EM, the mitochondrial biomass was observed. For each ROI per animal, 15 images were uniformly, and randomly chosen. Total mitochondrial area and density were calculated. Between the NAc-DBS group and the sham controls, the FC, PI, OCR/ECAR ratio, and mitochondrial biomass were compared using the Mann-Whitney test (p < 0.05).Results
The NAc-DBS group showed a noticeably higher FC in the mPFC, NAc, vHIPP, and VTA (Figure 1B). The normalized FC values were higher than the sham controls (Figure 2). In the NOR task, the PI of NAc-DBS group was higher (Figure 3). Figure 4A depicts the energy status following NAc-DBS, the NAc-DBS group displayed higher levels of energy than the sham controls. The OCR/ECAR ratio significantly increased in the NAc-DBS group, indicating that the mitochondrial function in dopaminergic pathway was enhanced (Figure 4B). In Figure 5A, the mitochondria in each ROI are displayed. Figures 5B & 5C quantify the size and density of mitochondria in the NAc-DBS group and sham controls. The size and density of the NAc-DBS group significantly increased, showing that NAc-DBS may have an impact on mitochondrial morphology.Discussion
After NAc-DBS, FC between the dopaminergic pathways was strengthened. Due to the capacity to modulate synaptic transmission and plasticity, dopamine neurons played a crucial part in memory processes14. Furthermore, previous study has demonstrated that the increased biomass of mitochondria would lead to a more energetic state15. As a result of increased FC and mitochondrial biomass in dopaminergic areas and vHIPP, cognitive performance was elevated after NAc-DBS.Conclusion
The findings suggested that NAc-DBS may be a possible therapeutic treatment for memory and metabolic disorders since improving energy metabolism and altering mitochondrial biomass in the dopaminergic pathway, which could enhance memory-related cognitive behavior.Acknowledgements
This work is financially supported by National Science and Technology Council under Contract numbers of MOST 111-2321-B-A49-005-, 111-2314-B-303-026-, 111-2221-E-A49-049-MY2, and 111-2314-B-038-059-MY3.
References
1. Aum DJ, Tierney TS. Deep brain stimulation: foundations and future trends. Frontiers in Bioscience-Landmark. 2018;23(7):162-82.
2. Lv Q, Du A, Wei W, Li Y, Liu G, Wang XP. Deep Brain Stimulation: A Potential Treatment for Dementia in Alzheimer's Disease (AD) and Parkinson's Disease Dementia (PDD). Frontiers in Neuroscience. 2018;12:360.
3. Demarin V, MOROVIĆ S. Neuroplasticity. Periodicum Biologorum. 2014;116(2):209-211.
4. Mallach A, Weinert M, Arthur J, Gveric D, Tierney TS, Alavian KN. Post mortem examination of Parkinson's disease brains suggests decline in mitochondrial biomass, reversed by deep brain stimulation of subthalamic nucleus. The FASEB Journal. 2019;33(6):6957-6961.
5. Love MR, Sripetchwandee J, Palee S, Chattipakorn SC, Mower MM, Chattipakorn N. Effects of biphasic and monophasic electrical stimulation on mitochondrial dynamics, cell apoptosis, and cell proliferation. Journal of Cellular Physiology. 2019;234(1):816-824.
6. Dong H-L, Wu H-Y, Tian Z-X, Luo Z, Wu Y-F, Zhao J. Electrical stimulation induces mitochondrial autophagy via activating oxidative stress and Sirt3 signaling pathway. Chinese Medical Journal. 2021;134(5):628.
7. Lee EJ, Oh JS, Moon H, et al. Parkinson Disease-Related Pattern of Glucose Metabolism Associated With the Potential for Motor Improvement After Deep Brain Stimulation. Neurosurgery. 2020;86(4):492-499.
8. de Araujo IE, Ren X, Ferreira JG. Metabolic sensing in brain dopamine systems. Sensory and Metabolic Control of Energy Balance. Springer; 2011:69-86.
9. Gebara E, Zanoletti O, Ghosal S, et al. Mitofusin-2 in the nucleus accumbens regulates anxiety and depression-like behaviors through mitochondrial and neuronal actions. Biological Psychiatry. 2021;89(11):1033-1044.
10. Floresco SB. The Nucleus Accumbens: An Interface Between Cognition, Emotion, and Action. Annual Review of Psychology. 2015;66(1):25-52.
11. Li S-J, Lo Y-C, Lai H-Y, et al. Uncovering the modulatory interactions of brain networks in cognition with central thalamic deep brain stimulation using functional magnetic resonance imaging. Neuroscience. 2020;440:65-84.
12. Wang Q, Ding S-L, Li Y, et al. The Allen mouse brain common coordinate framework: a 3D reference atlas. Cell. 2020;181(4):936-953.
13. K Soman S, Swain M, Dagda RK. Multiplexing Seahorse XF24 and ImageXpress Nano Platforms for Comprehensive Evaluation of Mitochondrial Bioenergetic Profile and Neuronal Morphology. Mitochondria. Springer; 2022:349-362.
14. Speranza L, di Porzio U, Viggiano D, de Donato A, Volpicelli F. Dopamine: The neuromodulator of long-term synaptic plasticity, reward and movement control. Cells. 2021;10(4):735.
15. Li S, Sheng Z-H. Energy matters: Presynaptic metabolism and the maintenance of synaptic transmission. Nature Reviews Neuroscience. 2022;23(1):4-22.
Figures
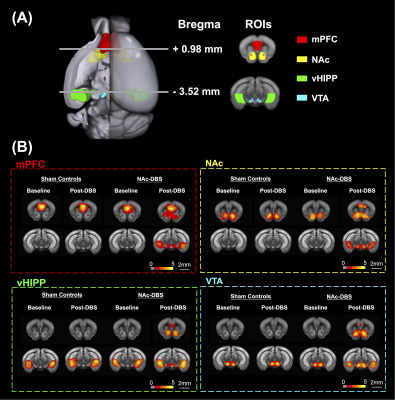
Figure 1. (A) Four ROIs in this study including mPFC (red), NAc (yellow), vHIPP (green), and VTA (blue). (B) The maps that corresponded to rsfMRI for different ROIs demonstrated on mPFC, NAc, vHIPP and VTA.
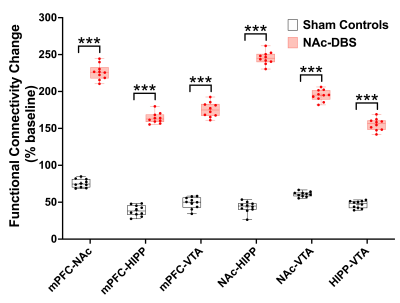
Figure 2. The mice in NAc-DBS group showed significant enhancement of FC compared with the sham controls after NAc-DBS. ***: p < 0.01
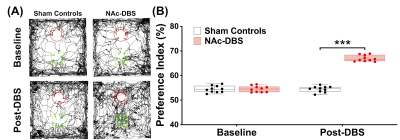
Figure 3. (A) The tracking visualization results showed the NAc-DBS group spent more time exploring novel object (green square) than familiar object (red circle). (B) After NAc-DBS, the PI in the NAc-DBS group become higher than sham controls. ***: p < 0.001
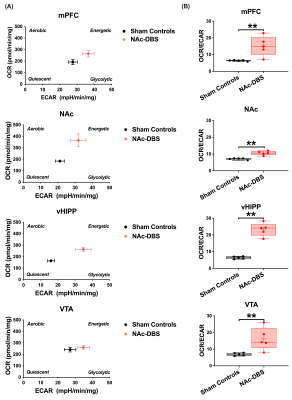
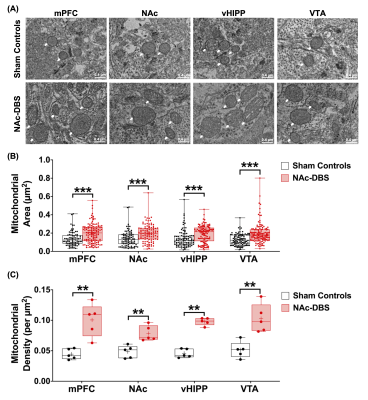
Figure 5. (A) Representative EM images of four ROIs in the sham controls and NAc-DBS group. Mitochondria was highlighted using white arrows. (B) Quantification of mitochondrial area from EM images in mPFC, NAc, vHIPP and VTA in the sham controls and NAc-DBS group. (C) Quantification of mitochondrial density from electron microscopy images in mPFC, NAc, vHIPP and VTA in the sham controls and NAc-DBS group. **: p < 0.01; ***: p < 0.001