1028
Examining the temporal evolution of top-down task dependent modulations across cortical depths.1CMRR, University of Minnesota, Minneapolis, MN, United States, 2CMRR, University of Minnesota, Mineapolis, MN, United States
Synopsis
Keywords: Brain Connectivity, Brain
We demonstrate the feasibility of simultaneously acquiring submillimeter and subsecond functional images to examine the temporal evolution of task-dependent modulations across cortical depths elicited by socially relevant stimuli such as faces at the single subject level. We achieved this using NORDIC denoising. Our results highlight the complexity of laminar fMRI results in humans which can be better resolved by high spatiotemporal recordings. We suggest that, when studied with sufficient, sub-second temporal resolution, depth-dependent fMRI has the potential to address laminar communication within and across areas, which is crucial to understanding the complexity of human hierarchal organization at the mesoscale level.Introduction
With the growing availability of UHF scanners and the continued optimization of accelerated acquisition protocols, the ability to record submillimeter functional images is becoming more accessible. At these resolutions it is possible to study the human brain at the mesoscale level, tackling some of the most fundamental units of neural computations such as Layers and Columns1. Invasive animal data have shown that feedforward inputs primarily terminate in middle layers while feedback or topdown connections occur in inner and outer layers. As the human fMRI field begins to shift from validations of animal results to uniquely human neuroscience questions, understanding the complexities of the human brain and its unique functional laminar architecture will become ever more important.Finer temporal resolution may represent one path to help unravel such complexity as it may reveal more information regarding neurovascular coupling and the specificity of the vascular response observed with GE-BOLD fMRI. While gains in SNR associated with higher field strength have been traditionally traded for increased spatial precision, recent evidence shows the benefits of high (i.e. sub-second) temporal resolutions, not only increasing statistical power, but also affording more precise insights in neuro-temporal dynamics2. However, acquiring functional images in humans with sub-millimeter and sub-second precision simultaneously is extremely challenging, requiring both in-plane and through plane accelerations. The resulting images are extremely low in SNR and thermal noise dominated, due to the combination of accelerations, fast TRs, and high spatial resolutions. While averaging across a large amount of data could make it feasible, lengthy data acquisitions are not ideal, especially when examining top down-related modulations across tasks3. Using BOLD fMRI to investigate task modulations4 , while fruitful, significantly increases the acquisition time, while decreasing the amount of data for averaging (as averaging needs to occur within tasks). Alternatively, thermal noise can be suppressed using NORDIC denoising5, an algorithm that suppresses thermal noise while leaving response amplitudes largely unchanged. Here we acquire BOLD images with sub-millimeter and sub-second resolutions while participants performed 2 different tasks to identical socially relevant face stimuli.Methods
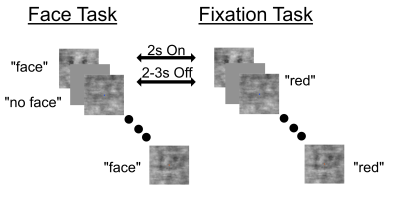
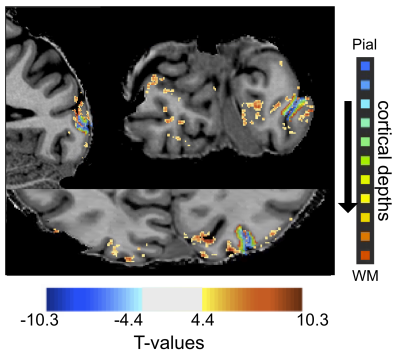
We used GE-EPI to acquire functional images (0.75 mm3 isotropic, TE = 24.6ms, flip angle =45.3°, 30 slices, TR= 739 ms, IPAT = 3, partial Fourier = 7/8, MB = 2) on a SIEMENS 7 Tesla scanner with a 1 Tx. 32 Rx. Nova coil. Flip angle was optimized based on B1+ maps in visual cortex and manual B0 shimming was performed to minimize distortion. We also acquired a resolution matched (0.75 mm3) T1 MP2RAGE. We used grayscale images of faces and manipulated the phase coherence of each face from 0% to 40% in 10% steps. Each stimulus was presented for 2 seconds followed by a pseudo random 2-3 second fixation. To increase jittering of stimulus presentation and allow for a better characterization of the HRF, 10 % of the trial were blank. A centrally presented fixation cross changing color every 250 ms, was held constant across tasks. Participants were instructed to perform either a stimulus-relevant face detection or a stimulus-irrelevant fixation color task. We acquired a total of 6 runs (each lasting approximately 4.5 minutes) and an additional run to localize the retinotopic representation of the stimuli in early visual cortices.Following manual segmentation of the cortex, we divided the cortical sheet into 11 equidistant depths, spanning the full distance from white matter to the pial surface. Independently per task and run we performed standard GLM analyses to deconvolve the timecourses in FIR curves (one per condition). We proceeded to average all voxels within a depth and compare the FIRs elicited by the face against those elicited by the fixation tasks.Results
As previously reported2,3,4, the stimulus-relevant face task elicited larger BOLD response relatively to the stimulus-irrelevant fixation task across all regions and depths. Consistently with previous findings4, we observed task modulations in inner depths of early visual cortices. The fine temporal sampling allowed observations of task modulations as early as 1.5 s (i.e. 2 TRs, p<.05) after stimulus onset, with the initial dip elicited by the fixation task being larger than that of the face task (as also reported in 2 albeit not at the cortical depth level). Unlike our previous report6, we further observe that these task-related modulations shift in time across depths, becoming more delayed towards the pial surface.Conclusion
This work demonstrates that it is feasible to simultaneously acquire BOLD images with sub-millimeter and sub-second resolutions with sufficient SNR to quantify depth dependent BOLD signal changes through time. In accordance with animal models, our results suggest that top-down modulations arrive in deep layers and move through the depths in a columnar-like fashion. The high quality of the data, enhanced by NORDIC denoising, allow performing single subject statistics and identifying the temporal evolution of topdown modulations across depths within participants.The data shown here, while preliminary, suggest that depth-dependent fMRI could resolve laminar communication within and across areas, which is crucial to understanding the complexity of human hierarchal organization at the mesoscale level. Simultaneous sub-second and sub-millimeter images also have the potential to resolve inconsistent results across laminar fMRI studies, which can be confounded by vascular effects and biases, further bridging the gap with invasive electrophysiology.Acknowledgements
E.Y. and L.V. were supported by the NIH grant Rf1 NIH Rf1MH116978References
1. De Martino, F. et al. The impact of ultra-high field MRI on cognitive and computational neuroimaging. Neuroimage 168, 366-382, doi:10.1016/j.neuroimage.2017.03.060 (2018).
2. Dowdle, L., Ghose, G., Chen, C.C., Ugurbil, K., Yacoub, E., Vizioli, L. (2021). Statistical Power or More Precise Insights into Neuro-Temporal Dynamics? Assessing the Benefits of Rapid Temporal Sampling in fMRI. Progress in Neurobiology
3. Dowdle, L., Ghose, G., Ugurbil, K., Yacoub, E. Vizioli, L. (2021) Clarifying the role of higher-level cortices in resolving perceptual ambiguity using ultra high field fMRI. Neuroimage
4. Dowdle, L., Ghose, G., Chen, C.C., Ugurbil, K., Yacoub, E., Vizioli, L. (2022) Using ultra-high field fMRI to uncover depth-dependent activation in the ventral temporal lobe. ISMRM
5. Vizioli, L., Moeller S., Dowdle, L., Akçakaya M., De Martino F., Yacoub E., Ugurbil K. (2021) Lowering the thermal noise barrier in functional brain mapping with magnetic resonance imaging. Nature Communications
6. Vizioli, L., Bratch, A., Ramanna, S., Ugurbil, K., Yacoub, E. (2019) Probing spatiotemporal information during attention modulations in humans with subsecond sampling of cortical depth dependent BOLD fMRI signals at 7T. ISMRM
Figures