1016
Selective MRI reacquisition to extend the working range of retrospective motion correction1Magnetic Resonance Section, DTU Health Tech, Technical University of Denmark, Kgs. Lyngby, Denmark, 2TracInnovations, Ballerup, Denmark, 3Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 4Department of Radiology, Harvard Medical School, Boston, MA, United States, 5Neurobiology Research Unit, Copenhagen University Hospital, Rigshospitalet, Copenhagen, Denmark, 6Department of Computer Science, University of Copenhagen, Copenhagen, Denmark
Synopsis
Keywords: Motion Correction, Artifacts
Retrospective motion correction (RMC) can substantially reduce motion artifacts in 3D brain MRI. However, for extensive motion, RMC performance is limited. We evaluate RMC with selective reacquisition (RMC+reacq) to expand the range of correctable motion, while directly comparing to prospective motion correction (PMC) for volumetric brain MRI, using external motion tracking. Both approaches lead to significant image quality improvement and the performance of RMC+reacq and PMC was only found statistically significant in 1 of 9 comparisons. These results suggest that RMC with selective reacquisition can match the performance of PMC for 3D-MPRAGE and 3D-FLAIR sequences.Introduction
Head motion remains a major challenge for brain MRI. Prospective and retrospective motion correction (PMC, RMC) aims to correct for motion during and after data acquisition, respectively. While PMC is most general, its dependence on MR-scanner’s complex real-time feedback architecture, and the “all-or-nothing” character of prospective correction can be major roadblocks for adoption. Using RMC, head rotation can cause Nyquist violations in k-space and spin history effects when confronted with extensive motion1, but RMC does not require high computational speed of motion estimation, preserves the original data, and can be implemented independent of scanner software.We evaluate the performance of RMC combined with selective reacquisition. We hypothesize that the reacquisition of data affected by the most severe pose changes will limit the remaining motion artifacts to a level manageable by RMC for 3D-MPRAGE and 3D-FLAIR sequences.
Methods
MRI and head motion tracking data were acquired from 22 healthy adults (16 females, 6 males) aged 23.5 ± 4.3 years. For details on recruitment and protocol please refer to 2,3. Data from 3 subjects were discarded due to inaccurate synchronization between the scanner and the external motion tracker needed for RMC. A total of 122 scans from 19 subjects were analyzed.Rigid-body head motion was measured with the optical motion tracking system Tracoline TCL 3.24 (TracInnovations, Ballerup, Denmark). MRI was performed using a 3T Siemens Magnetom Prisma scanner with a 64-channel head coil (Siemens Healthcare GmbH, Erlangen, Germany) and prototype sequences with PMC and selective reacquisition capabilities (3D-MPRAGE, 3D-FLAIR)5. Motion experiments consisted of a still reference scan, a scan with nodding motion (“YES”), and a scan with shaking motion (“NO”) (only for 3D-MPRAGE). Motion scans were repeated with and without PMC (“PMC-ON”, “PMC-OFF”).
After each acquisition, an additional 30 seconds was spent reacquiring the most motion-corrupted portions of k-space (16 k-space planes) based on a periodic motion score6. Original and reacquired data were corrected for motion using the same method (RMC or PMC).
PMC-OFF scans were retrospectively corrected for motion using pose estimates generated by the motion tracking system. For PMC, the tracking is smoothed with a 20-point moving median filter with a period of 670 ms. For RMC, tracking is smoothed with a 4-pole non-causal Butterworth low-pass filter with a cut-off of 0.5 Hz. All images were generated using the same off-scanner reconstruction pipeline.
Three image quality metrics were calculated for each corrected image using 7: structural similarity index (SSIM), peak signal-to-noise (PSNR), and Tenengrad (TGRAD) chosen based on superior correlation with radiologist scoring8. Average edge strength (AES) was also calculated but did not show any significant difference to uncorrected data independent of correction method and was therefore not included. Before calculation of quality metrics, all images were aligned to the reference image using sinc interpolation (SimpleITK rigid registration). For each subject, images were masked using a common brain mask derived from the uncorrected reference image (Freesurfer skull strip). For AES and TGRAD, the mask was used to crop image dimensions tightly around the brain, rather than region nulling, to avoid introducing false edges that can contribute to image-gradient-based metrics.
Statistical significance of quality metrics was evaluated with Wilcoxon signed rank test between no correction and each correction method, between RMC and PMC, and between RMC+reacq and PMC, compensated for multiple comparisons (false detection rate: 0.05).
Results
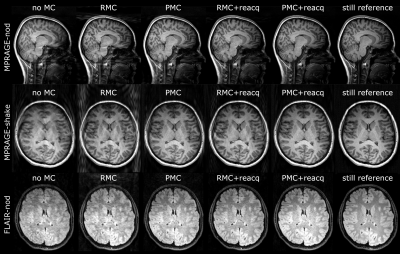
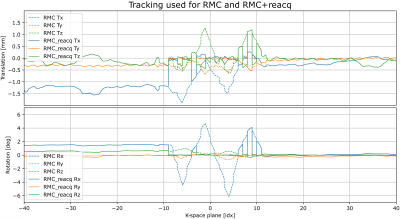
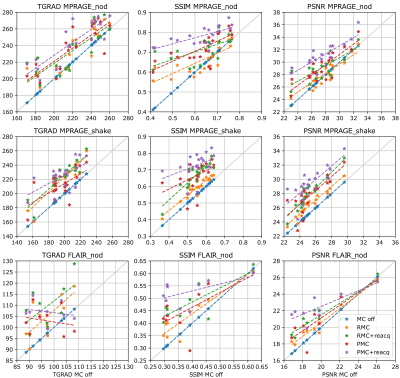
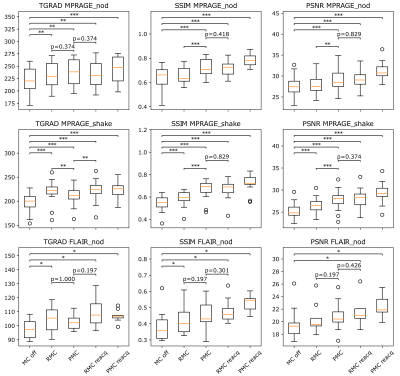
Figure 1 shows examples of each correction method for one patient. Figure 2 shows motion tracking with/without substituting reacquired data. Figure 3 provides quality metric data for each reconstructed image, and Figure 4 shows ranges and statistical significance for each quality metric difference.RMC+reacq, and PMC+reacq show significant improvement over no correction for all quality metrics and experiments, whereas RMC and PMC show significant improvement in all but three comparisons. RMC and PMC showed significant improvement in five comparisons. Comparison between RMC+reacq and PMC showed no significant difference except RMC+reacq being favored in one comparison.
Discussion
In this work, we based the comparison of two motion correction methods on volunteers reproducing prescribed motion patterns. While this pattern is not representative of all patient motion, it serves as a test case for the used methods favoring none of the correction approaches. The performance of RMC with reacquisition depends strongly on the specific characteristics and the duration of motion. The performance also depends on motion during the 30 second reacquisition period after the host sequence, which can be mitigated by gating the reacquisition with the motion tracking. In these motion experiments, the volunteers were always closer to their original position during the reacquisition period, which is not guaranteed in practice. The criteria for reacquisition used here are designed for PMC. A reacquisition criterion that seeks to fill undersampled regions of k-space might improve RMC+reacq performance and allow for shorter reacquisition times.Conclusion
Retrospective motion correction combined with selective reacquisition (RMC+reacq) can extend the working range of RMC by reducing the maximum motion magnitude affecting reconstructed data. In these motion experiments, RMC+reacq matched the performance of PMC for 3D-MPRAGE and 3D-FLAIR. The approach could reduce requirements of real-time computation and updating, and retain the original data while incurring a low penalty to overall scan time.Acknowledgements
No acknowledgement found.References
1. Slipsager JM, Glimberg SL, Højgaard L, et al. Comparison of prospective and retrospective motion correction in 3D-encoded neuroanatomical MRI. Magn. Reson. Med. 2022;87:629–645 doi: 10.1002/mrm.28991.
2. Eichhorn H, Frost R, Kongsgaard A, et al. Evaluating the performance of markerless prospective motion correction and selective reacquisition in a general clinical protocol for brain MRI. PsyArXiv. Nov 9, 2022. web: psyarxiv.com/vzh4g, doi: 10.31234/osf.io/vzh4g.
3. https://openneuro.org/datasets/ds004332/versions/1.0.1.
4. Olesen OV, Paulsen RR, Hojgaard L, Roed B, Larsen R. Motion Tracking for Medical Imaging: A Nonvisible Structured Light Tracking Approach. IEEE Trans. Med. Imaging 2012;31:79–87 doi: 10.1109/TMI.2011.2165157.
5. Frost R, Wighton P, Karahanoğlu FI, et al. Markerless high-frequency prospective motion correction for neuroanatomical MRI. Magn. Reson. Med. 2019;82:126–144 doi: 10.1002/mrm.27705.
6. Tisdall MD, Hess AT, Reuter M, Meintjes EM, Fischl B, van der Kouwe AJW. Volumetric Navigators (vNavs) for Prospective Motion Correction and Selective Reacquisition in Neuroanatomical MRI. Magn. Reson. Med. 2012;68:389–399 doi: 10.1002/mrm.23228.
7. https://github.com/melanieganz/MoCoProject/tree/main/ImageQualityMetrics.
8. Eichhorn H, Chemnitz-Thomsen S, Vouros E, et al. Evaluating the match of image quality metrics with radiological assessment in a dataset with and without motion artifacts. In: Joint Annual Meeting ISMRM-ESMRMB 2022. London, UK.
Figures