1005
Clinical reliability of the “Home Town Walk” fMRI paradigm for memory function localization in pre-surgical assessment of patients with Epilepsy1Medical Physics, University Hospitals Birmingham NHS Foundation Trust, Birmingham, United Kingdom, 2Radiology, University Hospitals Birmingham NHS Foundation Trust, Birmingham, United Kingdom
Synopsis
Keywords: Epilepsy, fMRI (task based), Memory function; Pre-surgical assessment; Clinical implementation; Clinical reliability
Memory-activated functional MRI (fMRI) is increasingly implemented in the clinic to assess memory function and inform pre-surgical decision making in refractory epilepsy. The Home Town Walking (HTW) fMRI paradigm has been shown to activate the parahippocampal gyri (PHG) and help determine memory lateralization in epilepsy patients. However, limited data are available on the reliability of this technique in clinical practice. This study aims to assess the robustness of the HTW paradigm for localising and lateralising memory function in a consecutive clinical series of 117 Temporal Lobe Epilepsy patients. Memory-related activation patterns were observed in 76% of cases with 94% reproducibility.Introduction
Memory-activated functional MRI (fMRI) is increasingly implemented in the clinic to assess memory function as part of pre-surgical decision making in refractory epilepsy1-5. The Home Town Walking (HTW) fMRI paradigm has been shown to activate the parahippocampal gyri (PHG) and help determine memory lateralization in epilepsy patients3, however limited data are available on the reliability of this technique. This study aims to assess the robustness of the HTW paradigm for lateralising memory function and aiding surgical planning in a large consecutive series of epilepsy patients.Methods
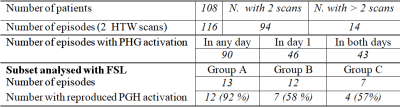
fMRI data were collected over 7 years (2015-2022) from 117 epilepsy patients across 2 Siemens 3T scanners (Verio and Skyra) using GE-EPI (3mm isotropic, TR=2s/3s) and a block-design HTW memory paradigm as part of a wider language and memory fMRI assessment. Patients were requested to participate in two fMRI sessions, usually less than 3 days apart (one episode) and were instructed to prepare an imagined but familiar walk prior to the first scanning session. Instructions were given to start and end at familiar places, divide the route into 10 blocks and list at least five memorable scenes or landmarks for each block. During the fMRI scans, patients were asked to visualise and recount their prepared detailed walk in their heads to the best of their abilities during the 30 s ‘Walk’ periods, and relax during the 30 s ‘Rest’ periods. High-resolution 3D T1-weighted scans were also acquired for anatomical reference.All data were analysed using a General Linear Model (GLM) with the manufacturer’s (Syngo VIA) BOLD processing package by a single experienced consultant neuroradiologist who produced a clinical report for surgical planning. The minimum cluster size and the t-statistical map threshold were subjectively varied to optimise a perceived balance between reliable PHG activation and spurious signals for each fMRI dataset. The clinical report included comments on the presence of PHG activation and if it was bilateral or predominantly left or right sided. The fused activation maps from all repeated fMRI sessions were compared and verified by MR physicists, and results compiled from the clinical reports to classify patients according to the reproducibility of the PHG activation pattern. A subset of 32 episodes (31 patients) were then selected from groups of patients who displayed reproducible (Group A) or non-reproducible (Group B) activation patterns and no activation (Group C) in either day within the PHG region. This subset of data were analysed using FSL FEAT (5mm kernel for spatial smoothing, z-score> 3.1, p-cluster=0.05). Activation maps were compared with the scanner generated maps and projected to a reference T1-weighted anatomical volume (collected on day 1) to compare activations across both days. Masks of posterior PHG right and left regions were created from Harvard-Oxford probabilistic atlas, projected to each patient’s reference space (using fnirt) and used to interrogate details of the statistical maps and compute Dice Coefficients (DC) across days within these regions.
Results
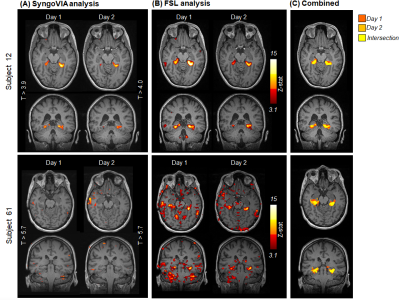
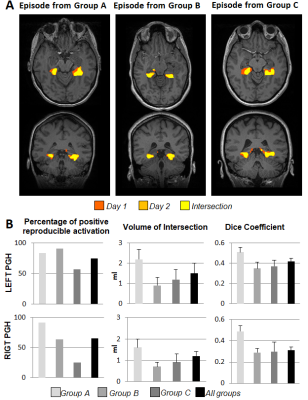
A total of 246 fMRI HTW datasets from 117 patients were assessed, of which 108 patients had repeated the scan at least twice (14 patients had additional follow up scans). BOLD activations were often observed in parahippocampal (PHG) and fusiform gyri, as well as language and visual areas. Figure 1 shows activation maps obtained with SyngoVIA and FSL for both days in two example subjects, and reproducibility on the combined FSL maps across days (intersection shown in yellow). Figure 2 summarizes the results: PHG BOLD activations were observed in at least one of the days for 90 (78%) of the episodes analysed with SyngoVia, with 93% of the PHG activation patterns evoked on day 1 successfully reproduced on day 2. Bilateral PHG activations were seen in 57% of these patients, with 34% and 9% predominantly left and right sided respectively. The additional FSL analysis on a subset of data revealed increased reproducibility of PHG activation patterns across days (when DC > 0.15), which was achieved for 72% of the analysed episodes. Figure 3 shows example of reproducible activation patterns for episodes across the three groups and details for each group of the percentage of reproducible activation, mean volume of the intersection and mean DC across activations from both days within the left and right PHG regions.Discussion & Conclusion
Despite being relatively difficult, most patients were able to perform the task given adequate preparation and reproducible PHG activations were achieved in the majority of cases. There was generally good agreement between the clinically processed results and the FSL analysis. However, the FSL analysis showed reproducible activity in 11 out of 17 cases where the clinical Syngovia analysis did not detect reproducible results. This was often the case when a higher threshold by the neuroradiologist due to stronger activation in other areas, as in subject 8 in Fig1, such that PHG regions were missed. FSL and Syngovia results are comparable when a lower t-stat threshold was used. Successful implementation of a repeated HTW paradigm shows reproducible (94% for positive studies) BOLD activations in medial temporal lobes sufficient to lateralise memory function in patients undergoing pre-surgical evaluation, which can be used as an adjunct to neuropsychological memory assessment. Future work will assess the correlation of fMRI results with postoperative memory outcome.Acknowledgements
No acknowledgement found.References
1. Avila C et al. Memory Lateralization with 2 Functional MR Imaging Tasks in Patients with Lesions in the Temporal Lobe. AJNR, 2006, 27:498-503.
2. Buck S & Sidhu MK. A Guide to Designing a Memory fMRI Paradigm for Pre-surgical Evaluation in Temporal Lobe Epilepsy. Front Neurol, 2020, 10:1354.
3. Janszky J et al., Functional MRI Predicts Memory Performance after Right Mesiotemporal Epilepsy Surgery . Epilepsia, 2005,46:244–250.
4. Towgood K, et al. Bringing Memory fMRI to the Clinic: Comparison of Seven Memory fMRI Protocols in Temporal Lobe Epilepsy. HBM, 2015,36:1595-608
5. Roland PE et al. Does mental activity change the oxidative metabolism of the brain? J Neurosci, 1987, 7:2373–89.
Figures