1000
Multi-tensor diffusion abnormalities of gray matter in an animal model of cortical dysplasia.1Instituto de Neurobiología, Universidad Nacional Autónoma de México, Querétaro, Mexico, 2Centro de Investigación en Matemáticas, Guanajuato, Mexico
Synopsis
Keywords: Epilepsy, Microstructure
Focal cortical dysplasias are characterized by abnormal cyto- and myelo- architecture and represent a frequent cause of epilepsy. In some cases these lesions are macroscopically subtle, often going undetected by several conventional imaging techniques, thus warranting the development of alternative imaging methods for their diagnosis. Novel methods for the analysis of diffusion-weighted imaging permit the investigation of the complex architecture of the cortex. Through spatial analysis of diffusion metrics using a multi-tensor approach we demonstrate abnormalities in an animal model of cortical dysplasia that reflect myeloarchitecture disarrangement as seen by histology.BACKGROUND
Focal cortical dysplasias (FCD) are malformations of cortical development characterized by cortical layer disruption and neuronal abnormalities that are associated with drug-resistant focal epilepsy 1–4. Surgical treatment is a viable option for some of these patients, with their outcome being highly related to complete surgical resection of lesions visible in magnetic resonance imaging (MRI). Unfortunately a high proportion of these lesions are subtle and difficult to detect by conventional imaging. Several methods to analyze MRI have been proposed, with the common goal of rendering subtle cortical lesions visible 3,5–8. However, most image-processing methods are targeted to detect the macroscopic characteristics of cortical dysplasias, which do not always correspond to the microstructural disarrangement of these cortical malformations. Quantitative analysis of diffusion-weighted MRI (dMRI) enables the inference of tissue characteristics, and novel methods provide valuable microstructural characteristics of complex tissue, including gray matter 9–11. Here we investigated the ability of advanced dMRI methods to detect diffusion abnormalities in an animal model of cortical dysplasia12.METHODS
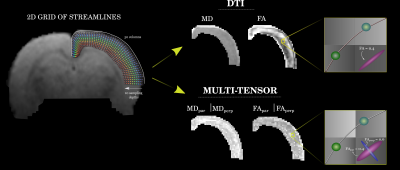
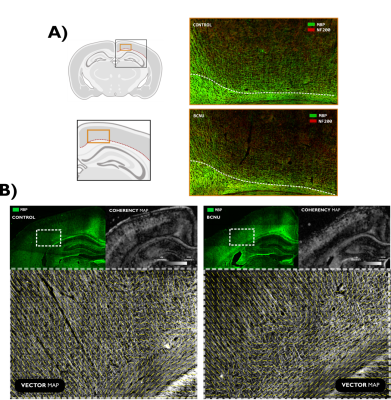
Pregnant Sprague-Dawley rats were intraperitoneally injected with either 1,3-Bis (2-chloroethyl) -1-nitrosourea (BCNU; 20 mg/kg) (n=4) or saline solution for control animals (n=4) at embryonic day 15 12. Resulting pups (BCNU: n=18; Control: n=19) were scanned in vivo at 30 postnatal days at the National Laboratory for MRI using a 7 T animal scanner and a 2x2 rat head array coil. Animals were anesthetized using isoflurane (4% for induction, 1.7% for maintenance) and vital signs were monitored. T2-weighted images were acquired with spatial resolution of 0.117 x 0.117 x 1.0 mm3 (TR/TE=4213/33 ms; 0.25 mm slice gap). dMRI were obtained with a pulsed gradient spin echo EPI sequence with diffusion sensitization in 90 different directions, each with b values of 670, 1270 and 2010 s/mm2 along with fifteen b=0 s/mm2 using an echo-planar encoding (EPI) sequence with spatial resolution of 0.175 x 0.175 x 1 mm3 (TR/TE=2000/22.86 ms; 0.25 mm slice gap). The dMRI data sets were first pre-processed with denoising13, removal of Gibbs-ringing artifacts14 and EPI susceptibility distortion correction15. For dMRI analyses we included the diffusion tensor (DTI),16,17 and the multi-resolution discrete search method (MRDS)18 that provides a multi-tensor representation of diffusion with independent diffusion profiles per axon bundle. To have a common anatomical descriptor of the cortex, we fitted a curvilinear 2D grid to the cortical ribbon as a coordinate system of streamlines following a Laplacian field19, each one with ten equidistant points spanning the entire depth of the cortex. Diffusion metrics were measured at each point in this curvilinear grid. Since MRDS fits one or more tensors at each voxel, resulting bundle-wise tensors were labeled as most parallel or most perpendicular to the streamlines spanning the depth of the cortex (Figure 1). Between-groups statistics were carried out using permutation tests with cluster inference. To explore the origin of diffusion abnormalities, we performed double immunofluorescence staining using primary antibodies for myelin basic protein (MBP; 1:200) and neurofilaments (NF200; 1:200). Images were acquired with an Apotome-Zeiss microscope and examined with structure tensor analysis.RESULTS
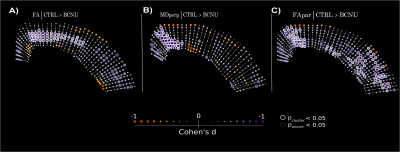
Assessment of brain macrostructure at 30 days of age using T2-weighted images showed normal morphological features in both groups. However, dMRI analyses revealed significant differences between groups. Fractional anisotropy derived from DTI was abnormally low at the level of motor cortex (M1, M2) and secondary somatosensory cortex (S1) in BCNU group. Multi-tensor metrics revealed further abnormalities of FA parallel-, and MD perpendicular to the streamlines. All results pointed to differences corresponding to the motor cortex and layers V-VI of the sensorimotor cortex (Figure 2). Immunofluorescence images highlighted abnormalities in the arrangement of tangential and radial fibers especially in the motor cortex. Structure tensor analysis revealed abnormalities of textural features derived from MBP and NF200 immunofluorescent images, showing loss of orientation and coherence of myeloarchitecture of deep cortical layers (Figure 3).DISCUSSION AND CONCLUSION
Our results suggest that dMRI is more sensitive than anatomical images for the identification of subtle cortical dysplasia in the early stages of development. Exploiting its ability to query mesoscopic environments, dMRI highlighted abnormalities of cortical architecture, particularly related to the coherence of intracortical myelinated fibers. Despite the use of methods tailored for the study of white matter and a relatively standard dMRI acquisition, MRDS proved useful for the identification of abnormalities and their separation of those related to ascending/descending fibers (i.e., parallel to the streamlines) and those related to tangential intra-cortical fibers (perpendicular to streamlines), thus providing an intuitive biological interpretation. Immunofluorescence stains coincide with the abnormalities in the MRDS metrics, highlighting a disorganized myeloarchitecture in experimental animals. Recent advancements in dMRI acquisition and biophysical modeling, such as the recent soma and neurite density (SANDI) model20 may further assess microstructure of gray matter. These findings suggest that the multi-tensor model (and other dMRI methods) could be explored as a potential approach for clinical applications.Acknowledgements
We thank Mirelta Regalado, Juan Ortiz-Rentana, Nydia Hernández-Ríos , Ericka de los Ríos, Gema Martínez, Leopoldo González-Santos for technical assistance. Funding provided by UNAM-DGAPA (IN2044720, IA200621).References
1. Blümcke, I. et al. The clinicopathologic spectrum of focal cortical dysplasias: A consensus classification proposed by an ad hoc Task Force of the ILAE Diagnostic Methods Commission 1. Int. Leag. Epilepsy Epilepsia 52, 158–174 (2010).
2. Guerrini, R. et al. Diagnostic methods and treatment options for focal cortical dysplasia. Epilepsia 56, 1669–1686 (2015).
3. Kabat, J. & Król, P. Focal cortical dysplasia - review. Pol. J. Radiol. 77, 35–43 (2012).
4. Moroni, R. F. et al. In vivo detection of cortical abnormalities in BCNU-treated rats, model of cortical dysplasia, using manganese-enhanced magnetic resonance imaging. Neuroscience 192, 564–571 (2011).
5. Spitzer, H. et al. Interpretable surface-based detection of focal cortical dysplasias: a Multi-centre Epilepsy Lesion Detection study. Brain (2022) doi:10.1093/brain/awac224.
6. Bonilha, L. et al. Voxel-based Morphometry Reveals Excess Gray Matter Concentration in Patients with Focal Cortical Dysplasia. Epilepsia 47, 908–915 (2006).
7. Bernasconi, A. et al. Texture analysis and morphological processing of magnetic resonance imaging assist detection of focal cortical dysplasia in extra-temporal partial epilepsy. Ann. Neurol. 49, 770–775 (2001).
8. Gill, R. S. et al. Multicenter Validation of a Deep Learning Detection Algorithm for Focal Cortical Dysplasia. Neurology 97, e1571–e1582 (2021).
9. Leuze, C. W. U. et al. Layer-Specific Intracortical Connectivity Revealed with Diffusion MRI. Cereb. Cortex N. Y. NY 24, 328–339 (2014).
10. McNab, J. A. et al. Surface Based Analysis of Diffusion Orientation for Identifying Architectonic Domains in the In Vivo Human Cortex. NeuroImage 69, 87–100 (2013).
11. Lorio, S. et al. MRI profiling of focal cortical dysplasia using multi‐compartment diffusion models. Epilepsia 61, 433–444 (2020).
12. Benardete, E. A. & Kriegstein, A. R. Increased Excitability and Decreased Sensitivity to GABA in an Animal Model of Dysplastic Cortex. Epilepsia 43, 970–982 (2002).
13. Veraart, J. et al. Denoising of diffusion MRI using random matrix theory. NeuroImage 142, 394–406 (2016).
14. Kellner, E., Dhital, B., Kiselev, V. G. & Reisert, M. Gibbs-ringing artifact removal based on local subvoxel-shifts. Magn. Reson. Med. 76, 1574–1581 (2016).
15. Andersson, J. L. R., Skare, S. & Ashburner, J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. NeuroImage 20, 870–888 (2003).
16. P J Basser. Estimation of the effective self-diffusion tensor from the NMR spin echo - PubMed. https://pubmed.ncbi.nlm.nih.gov/8019776/ (1994).
17. Veraart, J., Sijbers, J., Sunaert, S., Leemans, A. & Jeurissen, B. Weighted linear least squares estimation of diffusion MRI parameters: Strengths, limitations, and pitfalls. NeuroImage 81, 335–346 (2013).
18. Coronado-Leija, R., Ramirez-Manzanares, A. & Marroquin, J. L. Estimation of individual axon bundle properties by a Multi-Resolution Discrete-Search method. Med. Image Anal. 42, 26–43 (2017).
19. Lerch, J. P. et al. Cortical thickness measured from MRI in the YAC128 mouse model of Huntington’s disease. NeuroImage 41, 243–251 (2008).
20. Palombo, M. et al. SANDI: A compartment-based model for non-invasive apparent soma and neurite imaging by diffusion MRI. NeuroImage 215, 116835 (2020).
Figures