0996
A first application of the ILAE consensus protocol for 7T epilepsy imaging in addition to clinical practice1Department of Neurosurgery, Medical University of Vienna, Vienna, Austria, 2High-field MR Center, Department of Biomedical Imaging and Image-guided Therapy, Medical University of Vienna, Vienna, Austria, 3Christian Doppler Laboratory for MR Imaging Biomarkers, Vienna, Austria, 4Division of Neuroradiology and Musculoskeletal Radiology, Department of Biomedical Imaging and Image-guided Therapy, Medical University of Vienna, Vienna, Austria, 5Department of Neurology, Klinik Hietzing, Vienna, Austria, 6Center for Rare and Complex Childhood Onset Epilepsies, Member of ERN EpiCARE, Department of Pediatrics and, Medical University of Vienna, Vienna, Austria, 7Department of Neurology, Medical University of Vienna, Vienna, Austria, 8Division of Nuclear Medicine, Department of Biomedical Imaging and Image-guided Therapy, Medical University of Vienna, Wien, Austria
Synopsis
Keywords: Epilepsy, Brain
In a cohort of 38 patients with pharmacoresistant focal epilepsy, our implementation of the 2021 ILAE 7T consensus protocol found lesions in 19% of 3T MR-negative cases and improved delineation of lesions in 88% of 3T MR-positive cases, with statistically significant higher detection confidence at 7T. Our results conform to literature and show the surplus effect of the consensus protocol in addition to clinical standard diagnostics and point at a potential main use of better surgical planning in known lesions.Introduction
In patients with pharmacoresistant focal epilepsy1,2, resective or disconnective surgery is recommended3. Multiple investigations, e.g., long-term EEG-Video Monitoring, high-resolution MRI, and PET/SPECT, are needed to precisely define the epileptogenic zone4. 3T MRI is the current gold standard for detection/delineation of epileptogenic lesions, but remains negative/non-lesional in a third of cases5. In addition, some lesions, like mild cortical dysplasia or epilepsy-associated tumours are not well defined.7T MRI has been reported to increase sensitivity and specificity for the detection and delineation of epileptogenic lesions6,7. Thus, in 2021 the ILAE 7T Epilepsy Task Force proposed a consensus protocol5. This study evaluates the hypothesis that this protocol offers improved lesion detection as well as better lesion delineation in clinical practice.
Methods
After institutional review board approval, patients were recruited during pre-surgical evaluation at the Departments of Neurology and of Paediatrics. Inclusion criteria were age ≥ 12 years, informed consent by the patients and/or their legal guardians, and pharmaco-resistant focal epilepsy (either 3T non-lesional (MRN) or with suboptimal lesion delineation at 3T). Exclusion criteria were weight < 30 kg, claustrophobia, pregnancy, breastfeeding, and ferromagnetic implants.Presurgical evaluation including 3T MRI data following the HARNESS-MRI protocol8, Video-EEG monitoring (semiology at clinical seizure onset, ictal and interictal EEG) and [18F]FDG PET were used as a gold standard reference.
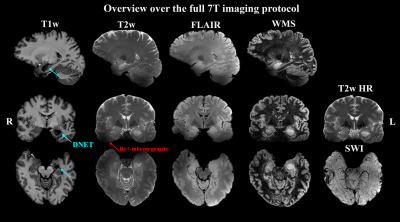
The protocol used a 7T scanner (Siemens Healthineers) with a 32-channel receive head coil (Nova Medical) and consisted according to the ILAE consensus protocol5 of 3D T1w (Tacq=8:02min, resolution=0.75×0.75×0.75mm³); 3D T2w (Tacq=7:02min, resolution= 0.7×0.7×0.7mm³); coronal hippocampal T2w (Tacq=6:07min, resolution= 0.5×0.4×2.0mm³); 3D T2w fluid-suppressed (Tacq=12:18min, resolution= 0.9×0.9×0.9mm³); 3D T2w fluid- and WM-suppressed (Tacq=3:54min, resolution= 0.9×0.8×1.0mm³); and transversal SWI (resolution=0.25×0.25×1.5mm³). The protocol required at least 50 min of measurement time.
MRI data were evaluated independently by two neuroradiologists specialised in epilepsy (rater 1: 16 years of epilepsy imaging experience; rater 2: 2 years of epilepsy imaging experience) three times (1=blinded 7T MRI, 2=blinded 3T MRI, 3=direct 3T 7T comparison). Parameters were presence of lesions (yes/no), identification confidence (scale 1-3) and lesion delineation (descriptive). Ratings were then aggregated, averaging confidence scores (0 used for no lesion detection). Lesion detection rates for 3T and 7T as well as inter-rater κ were calculated and confidence scores between 3T and 7T tested with a paired two-sided t-test. Percent scores for 7T lesion detection in 3T MRN cases, improvement in delineation, and overall cases with added benefits of 7T MRI to the clinical gold standard were calculated.
Results
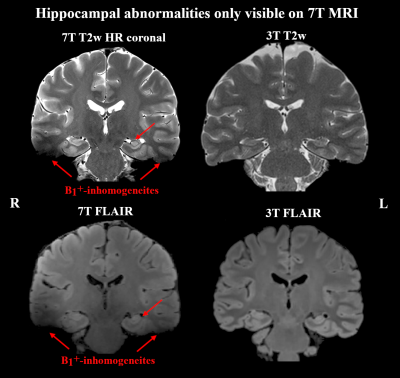
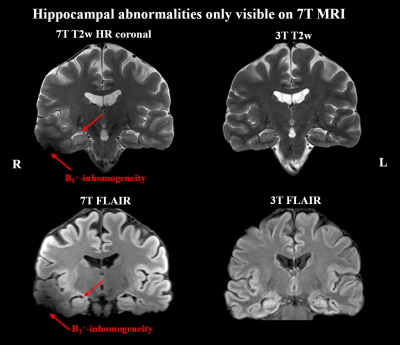
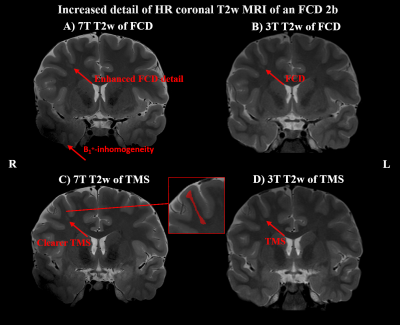
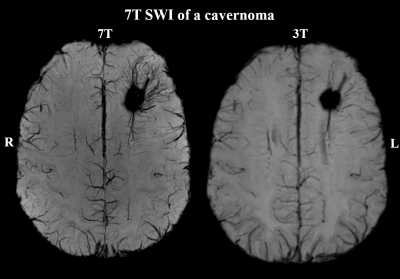
Of 41 recruited patients, 38 (age range 14-49 years, twenty female) completed the 7T protocol. The cohort included twenty-one 3T MRN cases and seventeen cases with insufficient clinical delineation. An example of 7T protocol quality is given in Figure 1. For 3T MRI, rater 1 reported a detection rate of 45% with a κ of 66% compared to 55% with a κ of 79% at 7. For the 22 patients with either 3T or 7T findings, mean confidence scores were 1.64±0.84 for 3T and 1.95±1.19 for 7T MRI (p=0.04).Additional 7T findings in 3T MRN patients were hippocampal abnormalities (three cases, examples in Figures 2+3) and focal cortical dysplasia (FCD) in one case, making up 19% of 3T MRN cases. 88% of the 17 patients with clinical 3T findings showed better delineation at 7T, as demonstrated for an FCD in Figure 4 and a cavernoma in Figure 5. Overall, in 50% of patients, potentially surgery-relevant information (e.g. additional findings or increased detail of known lesions) was found. B1+ inhomogeneities in the right temporal lobe were visible in all patients.
Discussion and Conclusions
Our study supports the newly released ILAE 7T consensus protocol recommendations by finding benefits in 50% of cases (with better delineation of 3T-visible lesions in the majority of cases). Compared with previous literature6,7,9,10, 19% new lesion detection rate in 3T MRN cases was lower, but this seems to be an effect of the inclusion of 1.5T MRI as reference in other studies. Our findings indicate that the addition of the 7T MRI consensus protocol to clinical routine already using modern 3T protocols would primarily improve the characterisation and delineation of epileptogenic lesions during presurgical evaluation.Our results are limited by a moderate cohort size and mono-centric study design. Standardised multi-center studies will be necessary to overcome this issue. In addition, our results show the need to address field inhomogeneities, e.g. by using parallel transmit systems.Acknowledgements
This study was supported by Neuroscience Cluster Seed Grants 2021 of the Medical University of Vienna Medical and the Medical-Scientific Fund of the Mayor of the Federal Capital Vienna (Project Number 21186).References
1. Ngugi AK, Bottomley C, Kleinschmidt I, Sander JW, Newton CR. Estimation of the burden of active and life-time epilepsy: A meta-analytic approach: Estimation of the Burden of Epilepsy. Epilepsia. 2010;51(5):883-890. doi:10.1111/j.1528-1167.2009.02481.x
2. Ryvlin P, Cross JH, Rheims S. Epilepsy surgery in children and adults. Lancet Neurol. 2014;13(11):1114-1126. doi:10.1016/S1474-4422(14)70156-5
3. Baumgartner C, Koren JP, Britto-Arias M, Zoche L, Pirker S. Presurgical epilepsy evaluation and epilepsy surgery. F1000Research. 2019;8:1818. doi:10.12688/f1000research.17714.1
4. Rosenow F. Presurgical evaluation of epilepsy. Brain. 2001;124(9):1683-1700. doi:10.1093/brain/124.9.1683
5. Opheim G, van der Kolk A, Bloch KM, et al. 7T Epilepsy Task Force Consensus Recommendations on the Use of 7T MRI in Clinical Practice. Neurology. 2021;96(7):327-341. doi:10.1212/WNL.0000000000011413
6. van Lanen RHGJ, Colon AJ, Wiggins CJ, et al. Ultra-high field magnetic resonance imaging in human epilepsy: A systematic review. NeuroImage Clin. 2021;30:102602. doi:10.1016/j.nicl.2021.102602
7. Park JE, Cheong EN, Jung DE, Shim WH, Lee JS. Utility of 7 Tesla Magnetic Resonance Imaging in Patients With Epilepsy: A Systematic Review and Meta-Analysis. Front Neurol. 2021;12:621936. doi:10.3389/fneur.2021.621936
8. Bernasconi A, Cendes F, Theodore WH, et al. Recommendations for the use of structural magnetic resonance imaging in the care of patients with epilepsy: A consensus report from the International League Against Epilepsy Neuroimaging Task Force. Epilepsia. Published online May 28, 2019:epi.15612. doi:10.1111/epi.15612
9. Veersema TJ, Ferrier CH, van Eijsden P, et al. Seven tesla MRI improves detection of focal cortical dysplasia in patients with refractory focal epilepsy. Epilepsia Open. 2017;2(2):162-171. doi:10.1002/epi4.1204110. Wang I, Oh S, Blümcke I, et al. Value of 7T MRI and post‐processing in patients with nonlesional 3T MRI undergoing epilepsy presurgical evaluation. Epilepsia. 2020;61(11):2509-2520. doi:10.1111/epi.16682
Figures