0968
Assessing Muscle Invasiveness In Bladder Cancer Based On Multiple Diffusion Models1College of Medical Imaging, Shanxi Medical University, Taiyuan, China, 2MR Scientific Marketing, Siemens Healthcare, Shanghai, China, 3Department of Radiology, The First Hospital of Shanxi Medical University, Taiyuan, China
Synopsis
Keywords: Diffusion/other diffusion imaging techniques, Cancer
Accurate assessment of the presence or absence of muscle invasiveness in bladder cancer is essential for selecting the best treatment options. In this study, we used 6 diffusion models including mono-exponential model (Mono), continuous-time random-walk (CTRW), incoherent motion within the voxel (IVIM), stretched exponential model (SEM), diffusion kurtosis imaging (DKI) and fractional-order calculus (FROC) model to assess muscle invasiveness in bladder cancer. The study showed that Mono,CTRW,IVIM,SEM and DKI could provide biomarkers for muscle invasion and distinguish non-muscle-invasive and muscle-invasive bladder cancer, and the combination of CTRW and FROC could further improve the classification accuracy.Introduction
Bladder cancer is the 11th most common malignancy worldwide, accounting for around 5.7 % of new cancer diagnoses and 1.9 % of cancer mortalities1 .As the first step in the management of bladder cancer, the level of muscle invasion must be evaluated for tumor staging and treatment planning. Muscle invasion is, currently, assessed mainly by transurethral resection (TUR) biopsy2 .However, TUR is invasive, and its potential to understage muscle invasion often prompts a second biopsy during the following treatment3 .As a non-invasive alternative, diffusion-weighted imaging (DWI) has been utilized as an imaging biomarker of tissue microstructure in bladder cancer4.Using a single parameter derived from DWI, e.g.,apparent diffusion coefficient (ADC), the study could predict muscle invasion of bladder tumor. Recently, studies using more advanced diffusion models found that parameters from fractional-order calculus (FROC), diffusion kurtosis imaging (DKI) and incoherent motion within the voxel (IVIM) could produce a more robust assessment of muscle invasion for bladder cancer5-7. In addition to FROC, DKI and IVIM, models such as mono-exponential model (Mono), continuous-time random-walk (CTRW), and stretched exponential model (SEM) have shown potential in characterizing pathophysiology for other malignancies ,e.g., hepatocellular carcinoma8 .The usefulness of these models, however, has not been investigated for bladder cancer for its aggressiveness by far. The aim of this study is to comprehensively evaluate and compare the efficacy of various parameters obtained from Mono, CTRW, IVIM, SEM, DKI and FROC models in distinguishing non-muscle-invasive bladder cancer (NMIBC) from muscle-invasive bladder cancer (MIBC).Materials and Methods
MR imaging: Between January 2022 to June 2022, sixty patients with bladder urothelial carcinoma were prospectively selected for the study. All patients underwent MRI first and within 3 months their malignancy were identified histopathologically by TUR of bladder tumor (TURBT). All MRI examinations were done at a 3T MRI scanner (MAGNETOM VIDA, Siemens Healthcare, Erlangen, Germany), and the details of parameters are shown in Table 1.Reconstruction & Segmentation: All DWI models: Mono, CTRW, IVIM, SEM, DKI and FROC were constructed using the data from the same DWI sequence, and post-processed using an in-house software BoDiLab, which was developed using Python 3.7. Region-of-interests (ROIs) was firstly drawn on DWI image that coverred the whole tumor by two radiologists independently (with 2 and 3 years of experience in body MRI, respectively) and then transferred to each individual diffusion parameter maps by rigid registration using Elastix. Patients were divided into two subgroups (Invasive vs. Non-invasive) based on their level of muscle invasion determined during TURBT.
Statistical Analysis:An independent two sample t test or Mann Whitney U test was applied to assess the differences of diffusion parameters between the two subgroups. Parameters were used as variables in a logistic regression model to make a binary group classification for each patient, and the accuracy of the classification was evaluated by a receiver operating characteristics (ROC) analysis. All analyses were done using SPSS software (version 25.0) and MedCalc (version 19.6.4) ) with a significant level set to p<0.05.
Results
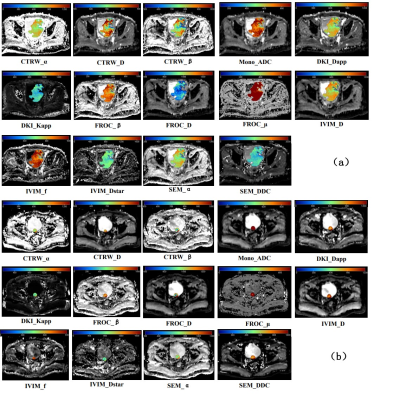
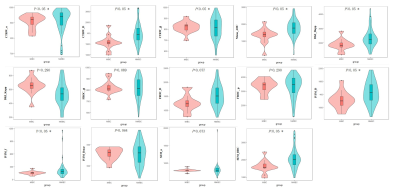
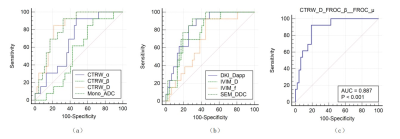
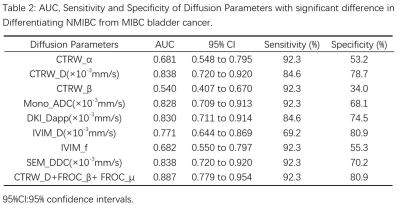
Out of 60 patients, TURBT identified 13 of them had muscle-invasive bladder cancer (MIBC), while 47 had non-muscle invasive bladder cancer (NMIBC). The maps of diffusion parameters including CTRW_α, CTRW_D, CTRW_β, Mono_ADC, DKI_Dapp, DKI_Kapp, FROC_β, FROC_D, FROC_μ, IVIM_D, IVIM_f, IVIM_Dstar, SEM_α and SEM_DDC from one patient with MIBC and one with NMIBC are shown in Figure 1. Among them, CTRW_α, CTRW_D, CTRW_β, Mono_ADC, DKI_Dapp, IVIM_D, IVIM_f and SEM_DDC were significantly higher for the NMIBC group compared to the MIBC group (Figure 2).Figure 3 and Table 2 show the ROC curves and the corresponding the areas under the receiver operating characteristic curve (AUC) of these parameters with CTRW_D and SEM_DDC having the highest AUC value of 0.838. When combined to create a multiparametric logistic regression model, CTRW_D, FROC_β, and FROC_μ together demonstrated a better group classification accuracy (AUC: 0.887, 95% CI:0.779 to 0.954 sensitivity:92.3, specificity: 80.9) than any single parameter models.
Discussion
It is known that, histopathologically, MIBC has a higher degree of tumor cellularity and tissue heterogeneity than NMIBC. These histopathological differences were likely reflected by the measurements from this study. First, CTRW_α, CTRW_D, CTRW_β, Mono_ADC, DKI_Dapp, IVIM_D, IVIM_f, and SEM_DDC are all inversely correlated with tissue cellularity5, 9,10, which explains why these parameters were all significantly higher in NMIBC than in MIBC. In addition, both CTRW and SEM models can reflect tissue heterogeneity by modeling multicompartmental water diffusion arising from the complex structure of tumor tissues11-13 ,and this could be why CTRW_D and SEM_DDC had the highest AUC in the classification between NMIBC and MIBC. The classification was further improved by combining multiple diffusion parameters: CTRW_D, FROC_β and FROC_μ, probably because that information about tumor cellularity, provided by CTRW_D and FROC_μ, and tissue heterogeneity, provided by FROC_β, were even better complemented after the combination.Conclusion
Our study showed that CTRW, Mono, DKI, IVIM and SEM could serve as noninvasive and quantitative biomarkers for muscle invasion in bladder cancer and the combination of CTRW and FROC could distinguish MIBC from NMIBC with a high accuracy, demonstrating that DWI is potentially a useful tool to individualize treatment strategies for bladder cancer.Acknowledgements
We thank Xiaochun Wang for guidance in the scanning program, whose important contributions to this study were indispensable to its success.References
1. SUNG H, FERLAY J, SIEGEL R L, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3): 209-249.
2. WITJES J A, BRUINS H M, CATHOMAS R, et al. European Association of Urology Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2020 Guidelines. Eur Urol. 2021; 79(1): 82-104.
3. AYATI M, AMINI E, SHAHROKHI DAMAVAND R, et al. Second Transurethral Resection of Bladder Tumor: Is it Necessary in All T1 and/or High-Grade Tumors?. Urology Journal. 2019; 16(2): 152-156.
4. KOBAYASHI S, KOGA F, YOSHIDA S, et al. Diagnostic performance of diffusion-weighted magnetic resonance imaging in bladder cancer: potential utility of apparent diffusion coefficient values as a biomarker to predict clinical aggressiveness. Eur Radiol. 2011; 21(10): 2178-2186.
5. FENG C, WANG Y, DAN G, et al. Evaluation of a fractional-order calculus diffusion model and bi-parametric VI-RADS for staging and grading bladder urothelial carcinoma. Eur Radiol. 2022; 32(2): 890-900.
6. ZHANG M, CHEN Y, CONG X, et al. Utility of intravoxel incoherent motion MRI derived parameters for prediction of aggressiveness in urothelial bladder carcinoma. Journal of Magnetic Resonance Imaging. 2018; 48(6): 1648-1656.
7. LI Q, CAO B, TAN Q, et al. Prediction of muscle invasion of bladder cancer: A comparison between DKI and conventional DWI. Eur J Radiol. 2021; 136: 109522.
8. GUO Y, CHEN J, ZHANG Y, et al. Differentiating Cytokeratin 19 expression of hepatocellular carcinoma by using multi-b-value diffusion-weighted MR imaging with mono-exponential, stretched exponential, intravoxel incoherent motion, diffusion kurtosis imaging and fractional order calculus models. Eur J Radiol. 2022; 150: 110237.
9. TANG L, ZHOU X J. Diffusion MRI of cancer: From low to high b-values. J Magn Reson Imaging. 2019; 49(1): 23-40.
10. WANG Y, HU D, YU H, et al. Comparison of the Diagnostic Value of Monoexponential, Biexponential, and Stretched Exponential Diffusion-weighted MRI in Differentiating Tumor Stage and Histological Grade of Bladder Cancer. Acad Radiol. 2019; 26(2): 239-246.
11. BENNETT K M, SCHMAINDA K M, BENNETT R T, et al. Characterization of continuously distributed cortical water diffusion rates with a stretched-exponential model. Magn Reson Med. 2003; 50(4): 727-734.
12. ALIZADEH A A, ARANDA V, BARDELLI A, et al. Toward understanding and exploiting tumor heterogeneity. Nature medicine. 2015; 21(8): 846-853.
Figures