0966
Permeability mapping in stroke via diffusion Standard Model with Exchange and S/V measurements from high-frequency OGSE1Champalimaud Research, Champalimaud Foundation, Lisbon, Portugal, 2Champalimaud Foundation, Lisbon, Portugal, 3Center of Functionally Integrative Neuroscience (CFIN) and MINDLab, Department of Clinical Medicine, Aarhus University, Aarhus, Denmark, 4Department of Physics and Astronomy, Aarhus University, Aarhus, Denmark
Synopsis
Keywords: Contrast Mechanisms, Microstructure
Membrane permeability plays an important role in numerous physiological processes ranging from cell volume regulation to neural activity. Until now, diffusion MRI measurements mainly provided information on exchange rates; however, to map permeability, information on the surface-to-volume (S/V) ratio is needed. Here, we apply ultrahigh frequency OGSE to infer on S/V from the associated power law dependence (1/ω1/2,) and the Standard Model with Exchange (SMEX) model for extracting the neurite -> extracellular space exchange rates in stroked rat brains. Combined, we present quantitative measurements of permeability which show plausible values and striking contrast in stroke.Introduction
Exchange is (re)emerging as an important contributor to diffusion MRI (dMRI) signals, especially in gray matter, whose microstructural characterization remains elusive. The diffusion Standard Model with Exchange (SMEX)1 and the Neurite Exchange Imaging2 models provide insights into exchange by observing characteristic signatures in dMRI signal decays. While SMEX reports neurite-to-extracellular space exchange rates, a critical step is to advance from exchange rates to permeability. Given an exchange rate rN between neurites with fraction fN and extracellular space, the permeability P in the barrier limited exchange regime is given as3–6:Eq.1 $$P = f_N r_N×V/S$$
where V/S represents the inverse of the surface-to-volume (S/V) ratio3–6.
Interestingly, S/V can be obtained from D(ω) in oscillating gradient spin echo (OGSE) experiments8 if the gradient oscillating frequency ωOGSE > D0/lc2 where lc is the microstructural restriction length scale and D0 is the intrinsic diffusivity. In this regime,
Eq.2 $$D(\omega)=Re\left\{\mathfrak{D(\omega)}\right\}=D_0\left(1-\frac{c(N)}{3\sqrt{2}}\frac{2S}{V}\sqrt{\frac{D_0}{\omega}}\right)$$
where c(N) is a numerical factor depending on the number of oscillations8 and the factor of two in 2S/V accounts for both the extra- and intra-neurite part of S/V7. Although Eq. 2 was derived for impermeable pores, it also describes the behavior for large ω for finite permeability, since exchange effects only appear in higher order terms9.
Thus, combining fN and rN from SMEX measurements and S/V from OGSE experiments at sufficiently high frequencies can provide an estimate of P from Eq. 1.
Methods
All animal experiments were preapproved by institutional and national authorities and carried out according to European Directive 2010/63.Stroke Induction and sample preparation. N=3 adult mice underwent stroke induction using an established protocol10. After 3h, the animals were transcardially perfusion fixed; brains were extracted and kept in fixative for 48h, washed in PBS for 48h, and then placed in a 10mm NMR tube filled with Fluorinert.
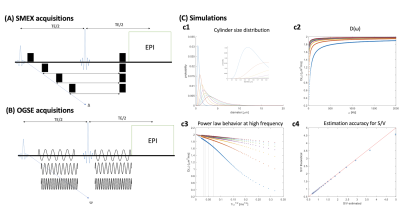
MRI. All MRI experiments were performed on a Bruker Aeon Ascend 16.4T scanner equipped with a Micro5 probe capable of delivering up to 3000 mT/m. Samples were kept at 37°C during diffusion experiments, which were all acquired with EPI (384 kHz bandwidth, 2 double-sampled shots, 1.38 pFT undersampling), TR/TE = 3000/54 ms, in-plane resolution 100×100 µm2, slice thickness = 600 µm.
SMEX experiments were acquired with 30 b0s, 32 b-value shells up to 60 ms/µm2, 30 directions per shell, δ=6ms, and 8 different s ranging from 8.5-35 ms.
OGSE experiments were acquired with cosine modulated waveforms, 30 b0s, 1 b-value of 0.5 ms/µm2, 30 directions, and 30 ω values ranging from 25-730 Hz with number of oscillations correspondingly ranging between 1 and 30 with for each half waveform duration of 20.5 ms.
Data Analysis. Tensor MPPCA11 denoising was applied prior to reconstruction of EPI data. SMEX parameters, including neurite fraction and exchange rate, were extracted as in Olesen et al1. OGSE data was fitted to a simple diffusion tensor and the mean diffusivity was plotted as 1/√ω, and then was linearly fit to Eq. 2.
Simulations. Simulations for the linear oscillation frequency regime in the presence of small neurites was performed by simulating lognormal cylindrical size distributions and their respective D(ω) using Stepisnik’s expressions12 with D0 = 2 µm2/ms. D(ω) was then plotted against 1/√ω, the linear regime examined, and S/V was extracted and compared against ground truth.
Results
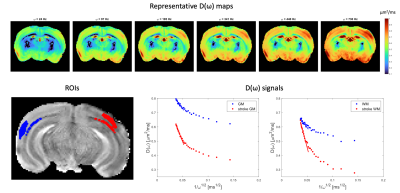
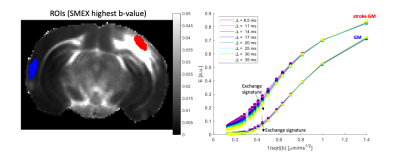
Simulations revealed that even for small cylinder size distributions, oscillation frequencies of >400Hz maintain the linear regime (Figure 1C). The S/V extracted for most distributions is in good agreement with the ground truth, and even for the smallest distributions the error is < 15 % in S/V.D(ω) maps derived from OGSE experiments in a representative slice (Figure 2) reveal the expected increase in diffusivity with oscillation frequency. ROIs placed in contralateral GM and stroked GM show a relatively linear regime between 400 – 750 Hz. In contralateral WM, the linear regime is less obvious, while in stroked WM the linear regime is clearly achieved. Figure 3 shows ROIs in SMEX experiments. The signals clearly reveal the exchange signatures.
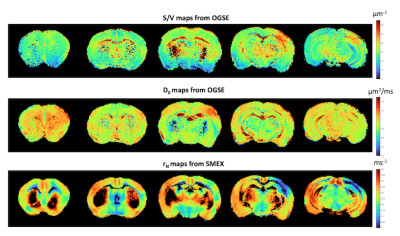
Figure 4 presents the S/V and D0 maps derived from fitting the OGSE-driven voxelwise to Eq. 2 and SMEX-derived exchange maps. Strikingly, S/V increases in stroke, and exchange rate decreases in stroke tissue. Note that in the stroke periphery, S/V remains quite comparable to contralateral values while D0 increases, potentially suggesting a different type of edema in these areas.
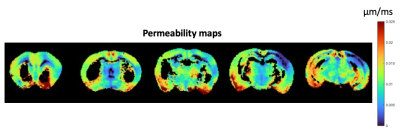
Figure 5 presents water permeability maps in the stroked brain (masked for areas clearly not adhering to the SMEX model). P is within reasonable ranges of ~0.015 µm/ms in normal GM tissue. In stroke, a clear decrease in permeability is observed. The results were consistent across 3 brains.
Discussion
The permeability maps presented here incorporate complementary information from very high-frequency OGSE and SMEX. The higher S/V in the stroke core is consistent with beaded neurites10,13,14 and the decreased permeability likely represents metabolic failure upon ischemia.Conclusion
Permeability mapping was presented and the first applications in stroke bode well for future applications and better understanding of biophysical properties of tissues.Acknowledgements
CONGENTO, PORTUGAL 2020 European Regional Development Fund (ERDF) and Fundação para a Ciência e Tecnologia LISBOA-01-0145-FEDER-022170.References
1. Olesen J, Østergaard L, Shemesh N, Jespersen S. Diffusion time dependence, power-law scaling, and exchange in gray matter. Neuroimage. 2022; 251:118976.
2. Jelescu I, de Skowronski A, Geffroy F, et al. Neurite Exchange Imaging (NEXI): A minimal model of diffusion in gray matter with inter-compartment water exchange. Neuroimage. 2022; 256:119277.
3. Mitra P, Sen P, Schwartz L. Short-Time Behavior of the Diffusion Coefficient as a Geometrical Probe of Porous Media. Physical Review B. 1993; 47:14.
4. Reynaud O. Time-dependent diffusion MRI in cancer: Tissue modeling and applications. Front Phys. 2017;
5. Ash B, Barrer R, Craven R. Sorption Kinetics and Time-Lag Theory Part 1 .-Constant Diffusion Coefficient. Journal of the Chemical Society. 1978
6. Kärger J. NMR self-diffusion studies in heterogeneous systems. Adv Colloid Interface Sci. 1985; 23:129-148.
7.Latour L, Svoboda K, Mitra P, Sotak C. Time-dependent diffusion of water in a biological model system. Proc Natl Acad Sci U S A. 1994;91(4):1229-1233.
8. Reynaud O, Winters K, Hoang D, Wadghiri Y, Novikov D, Kim S. Surface-to-volume ratio mapping of tumor microstructure using oscillating gradient diffusion weighted imaging. Magn Reson Med. 2016;76(1):237-247.
9. de Swiet T, Sen P. Time dependent diffusion coefficient in a disordered medium. Journal of Chemical Physics. 1996;104(1):206-209.
10. Alves R, Henriques R, Kerkelä L, Chavarrías C, Jespersen S, Shemesh N. Correlation Tensor MRI deciphers underlying kurtosis sources in stroke. Neuroimage. 2022; 247.
11. Olesen J, Ianus A, Østergaard L, Shemesh N, Jespersen S. Tensor denoising of multidimensional MRI data. Magnetic Resonance in Medicine. 2022, 1-13.
12. Stepihik J. Time-Dependent Self-Diffusion by NMR Spin-Echo. Physica B. 1993; 183.;
13. Budde M, Frank J. Neurite beading is sufficient to decrease the apparent diffusion coefficient after ischemic stroke. Proc Natl Acad Sci U S A. 2010;107(32):14472-14477.
14. Skinner N, Kurpad S, Schmit B, Budde M. Detection of acute nervous system injury with advanced diffusion-weighted MRI: a simulation and sensitivity analysis. NMR Biomed. 2015;28(11):1489-1506.
Figures