0962
Tackling the gyral bias and bottleneck problems with hybrid diffusion-microscopy tractography in the BigMac dataset1Wellcome Centre for Integrative Neuroimaging, FMRIB, Nuffield Department of Clinical Neurosciences, University of Oxford, Oxford, United Kingdom, 2Wellcome Centre for Integrative Neuroimaging, Experimental Psychology, University of Oxford, Oxford, United Kingdom, 3Department of Oncology, University of Oxford, Oxford, United Kingdom, 4Donders Institute for Brain, Cognition and Behaviour, Radboud University Nijmegen, Nijmegen, Netherlands, 5Nuffield Department of Clinical Neurosciences, University of Oxford, Oxford, United Kingdom
Synopsis
Keywords: Tractography & Fibre Modelling, Multimodal, diffusion MRI, microscopy
We present data fusion combining precisely co-registered dMRI and microscopy to reconstruct 3D fibre orientation with micron-scale spatial resolution. We then perform hybrid dMRI-microscopy tractography to investigate two known challenges in tractography: the gyral bias and bottleneck problems. Our results using hybrid tractography suggest that the gyral bias can be overcome by increased spatial resolution, and that microstructure-informed fibre orientations can overcome the bottleneck problem, irrespective of spatial resolution. These observations can be used to inform tractography analyses in vivo. This approach builds on the complementary strengths of microscopy (high spatial resolution) and dMRI (3D whole-brain coverage).
Introduction
Obtaining accurate connectivity estimates from diffusion tractography is challenging due to issues such as the gyral bias1 and bottleneck2 problems. In gyral bias, partial volume effects cause streamlines to preferentially terminate at gyral crowns rather than capturing sharp turns into gyral walls. In bottleneck regions, multiple fibre bundles converge with parallel orientation causing streamlines to become indistinguishably mixed. Both challenges are caused by partial volume effects at limited resolution.These challenges are particularly problematic for complex white matter architecture in primate brains, including humans. Complementary methods at microscopic resolution could inform dMRI tractography, but are currently limited: (i) chemical tracers provide a direct estimate of axonal projections but are limited to a single/few injection(s) per animal3,4; (ii) microscopy stains or polarized light imaging (PLI) can estimate fibre orientation but only in 2D; (ii) 3D PLI has been demonstrated but with very limited coverage5,6,7. Consequently, none of these methods currently provide dense reconstruction of white matter tracts across whole primate brains.
We approach this challenge through a combination of a unique dMRI-microscopy dataset, BigMac8, and a novel hybrid dMRI-microscopy tractography approach9. The hybrid method informs 2D orientations estimated from microscopy with 3D orientation distributions from dMRI to obtain whole brain, 3D orientations at high spatial resolution. The hybrid orientations can be combined into fibre orientation distributions (FODs) at arbitrary resolutions for use in tractography.
Our previous works have explored hybrid tractography9 using PLI over small regions of interest (ROIs). Here we extend the analysis to three different microscopy contrasts (PLI, myelin- and Nissl-stained histology), perform whole brain tractography at multiple spatial resolutions, and investigate whether improved spatial resolution and microscopy-informed fibre orientations can overcome the gyral bias or bottleneck problems.
Methods
The BigMac brain was scanned postmortem with dMRI and structural MRI protocols. The brain was then sectioned along the anterior-posterior axis, with slices assigned to microscopy contrasts including i) PLI, ii) Gallyas silver staining of myelin and iii) Cresyl violet staining of cell bodies. Each contrast is repeated every 350μm throughout the brain. MRI-microscopy co-registration was performed using TIRL10.Hybrid microscopy-dMRI orientations9 were estimated for each microscopy pixel, where microscopy provided the in-plane orientation and dMRI approximated the through-plane orientation (Fig. 1). This resulted in 3D fibre orientations at the resolution of the microscopy. The orientations were then combined into spherical harmonic FODs at the resolution of 0.4, 0.6 and 1mm. For the central region of the BigMac brain that is missing microscopy (~3 dMRI voxels thick where the brain was sectioned in two anterior and posterior halves), FODs were estimated solely from the dMRI using CSD11.
Whole brain probabilistic tractography of 42 tracts was implemented with MRtrix312 using masks from XTRACT13. Streamlines were removed if they did not overlap well with the population-based probabilistic mask from XTRACT (defined as the mean probability of all the voxels the streamlines intersect being lower than 0.2). Streamlines 4 standard deviations larger than the mean fibre length and 3 standard deviations away from the fibre core were discarded14.
We studied a bottleneck region within the motor network. The functional representation of the trunk, arms and face areas are distributed across the motor cortex in a topographic latero-medial pattern15. We investigate whether hybrid tractography with different spatial resolutions can retain this topographic organisation as fibres enter the internal capsule (IC) on their way to the rest of the body. ROIs were manually drawn on the structural image (Fig. 5) and used for tractography seed masks. Density maps within the IC were obtained for each projection. The gyrus bias problem was studied with probabilistic tractography seeding from the white matter, and the streamline distribution at the gyrus was visualized.
Results
Figure 2 demonstrates how the hybrid FODs and resulting tractography from the different microscopy contrasts follow our neuroanatomical expectations, giving confidence in the generalisability of our method. Interestingly, histological stains estimate considerably more crossing-fibre voxels than PLI.Figure 3 demonstrates the whole-brain microscopy-informed reconstruction of tracts. The cortico-spinal tract (CST) is fairly well aligned with the microscopy plane where microscopy is most informative. In comparison, the optic radiation primarily extends AP i.e. through the microscopy plane. The hybrid method is able to reconstruct both, and resolve branching fibres such as those extending from the CST to more lateral cortical regions.
Figure 4 demonstrates how increased spatial resolution in hybrid tractography can delineate expected gyral patterns of fibre fanning and overcome the gyral bias problem which is present at 1mm.
Figure 5 demonstrates how microstructure-informed fibre orientations can be used to overcome the bottleneck problem within the IC, irrespective of the spatial resolution. The hybrid method consistently retains the strong topographic organisation of streamlines through the bottleneck region. In the dMRI data, the projection of streamlines from each ROI becomes mixed.
Future Work
The hybrid tractography is generalizable to other multi-modal datasets and code will be made open access on GitHub. Future work will examine the gyrus bias and bottleneck regions across the whole brain with the aim of quantitatively identifying canonical modes of failure, which can inform on more advanced tract reconstruction in vivo (e.g. by using the microscopy data as priors for machine learning methods).Acknowledgements
AFDH and KLM contributed equally to this work. SZ is supported by the Chinese Government Scholarship and the Nuffield Department of Clinical Neurosciences studentship. KLM, AFDH, MC, and SJ are supported by the Wellcome Trust (grants WT202788/Z/16/A, WT215573/Z/19/Z, and WT221933/Z/20/Z). The Wellcome Centre for Integrative Neuroimaging is supported by core funding from the Wellcome Trust (203139/Z/16/Z). RBM is supported by the EPA Cephalosporin Fund and Biotechnology and Biological Sciences Research Council (BB/N019814/1) grants.
References
- Schilling K, et al. Confirmation of a gyral bias in diffusion MRI fiber tractography. Hum Brain Mapp. 2018
- Schilling KG, et al. Prevalence of white matter pathways coming into a single white matter voxel orientation: The bottleneck issue in tractography. Hum Brain Mapp, 2022
- Gao L, et al. Single-neuron projectome of mouse prefrontal cortex. Nat Neurosci, 2022
- Oh SW, et al. A mesoscale connectome of the mouse brain. Nature, 2014
- Larsen L, et al. Polarized light imaging of white matter architecture. Microscopy Research and Technique, 2007
- Schubert N, et al. 3D Polarized light imaging portrayed: visualization of fiber architecture derived from 3D-PLI. High-Resolution Neuroimaging, 2017
- Axer M, et al. High-resolution fiber tract reconstruction in the human brain by means of three-dimensional polarized light imaging. Frontiers in Neuroinformatics, 2011
- Howard AF, et al. The BigMac dataset: an open resource combining multi-contrast MRI and microscopy in the macaque brain. bioRxiv, 2022
- Howard AF, et al. The microscopy connectome: towards 3D PLI tractography in the BigMac dataset. ISMRM 28th Annual Meeting, 2020
- Huszar IN, et al. Tensor Image Registration Library: Automated Deformable Registration of Stand-Alone Histology Images to Whole-Brain Post-Mortem MRI Data. bioRxiv, 2022
- Tournier JD, et al. Robust determination of the fibre orientation distribution in diffusion MRI: Non-negativity constrained super-resolved spherical deconvolution. NeuroImage, 2007
- Tournier JD, et al. MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. NeuroImage, 2019
- Warrington S, et al. XTRACT - Standardised protocols for automated tractography in the human and macaque brain. Neuroimage, 2020
- Yeatman JD, et al. Tract profiles of white matter properties: automating fiber-tract quantification. PLoS One, 2012
- Park MC, et al. Consistent features in the forelimb representation of primary motor cortex in rhesus macaques. J Neurosci, 2001
Figures
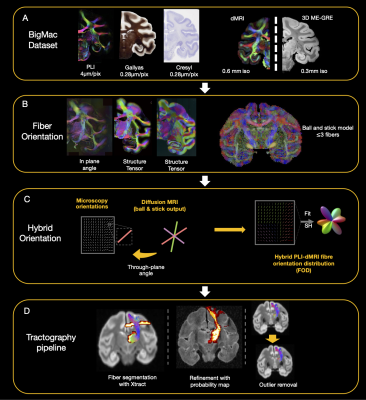
Figure 1: Overview of the dataset and analysis pipeline. A. BigMac includes co-registered microscopy (PLI, myelin- and Nissl-staining) and postmortem MRI (dMRI/structural). B. Fibre orientations were extracted from each microscopy contrast (in-plane angle from PLI, structure tensor analysis from histology) and dMRI (ball and stick model). C. Hybrid orientations are generated with the in-plane orientation from microscopy and through-plane orientation from dMRI. D. The tractography results are optimized with XTRACT probability maps and outlier removal.
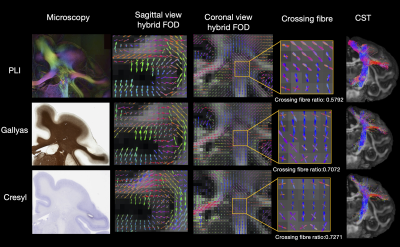
Figure 2: Hybrid fibre orientation distributions (FODs) reconstructed at 0.6mm resolution from different microscopy contrasts: PLI, myelin- and Nissl-stained histology (Gallyas/Cresyl). All three contrasts show fibre orientations aligned with our neuroanatomical expectations. Noticeably, the histology FODs depict more multi-fibre voxels (crossing fibre ratio = Nwm,multi-fibre / Nwm,voxels with fibre) These FODs can be fed into tractography to reconstruct white matter tracts (example CST shown).
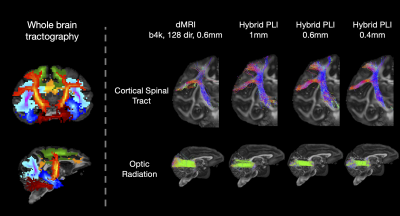
Figure 3: Whole brain microscopy-informed tractogram reconstructed from hybrid orientations. The probability maps for different tracts reconstructed from hybrid PLI-MRI at 0.6mm are shown (Left). The hybrid method can successfully reconstruct numerous tracts throughout the brain including the CST, an example tract within the coronal plane where microscopy is the most informative (top), and the optic radiation, a tract extending primarily along the anterior-posterior axis where dMRI provides most information (bottom).
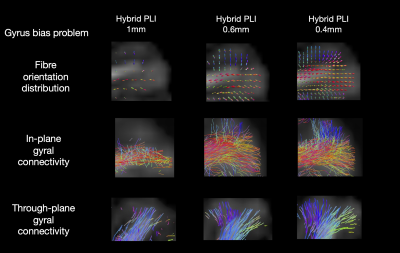
Figure 4: The gyral bias problem. FODs (top) and tractography of two gyri which primarily lie within (middle) and through (bottom) the microscopy plane. Outputs are shown for hybrid PLI-dMRI using the same underlying data, but FODs reconstructed at different resolutions. The gyral bias problem is present in the 1mm, whilst the high spatial resolution of 0.4mm successfully delineates the fibre fanning.
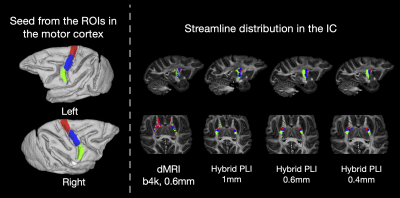
Figure 5: The bottleneck problem. ROIs relating to the functional representation of the trunk, arm and face regions (Left). Tractography was seeded from the ROIs to reconstruct streamlines passing through the internal capsule (IC). The bottleneck problem is observed in the dMRI as streamlines from the ROIs are mixed. With the hybrid method, the streamlines from each ROI demonstrate a clear anterior-posterior distribution in the bottleneck region. Notably, the ROIs are separable at all resolutions.