0958
Deep learning reconstruction of radial T2 weighted data sets with data consistent unrolled neural networks
Brian Toner1, Simon Arberet2, Fei Han3, Mariappan Nadar2, Vibhas Deshpande4, Diego Martin5, Maria Altbach6, and Ali Bilgin7,8
1Applied Mathematics, The University of Arizona, Tucson, AZ, United States, 2Digital Technology & Innovation, Siemens Healthineers, Princeton, NJ, United States, 3Siemens Healthineers, Los Angeles, CA, United States, 4Siemens Healthineers, Austin, TX, United States, 5Radiology, Houston Methodist, Houston, TX, United States, 6Medical Imaging, The University of Arizona, Tucson, AZ, United States, 7Electrical and Computer Engineering, The University of Arizona, Tucson, AZ, United States, 8Biomedical Engineering, The University of Arizona, Tucson, AZ, United States
1Applied Mathematics, The University of Arizona, Tucson, AZ, United States, 2Digital Technology & Innovation, Siemens Healthineers, Princeton, NJ, United States, 3Siemens Healthineers, Los Angeles, CA, United States, 4Siemens Healthineers, Austin, TX, United States, 5Radiology, Houston Methodist, Houston, TX, United States, 6Medical Imaging, The University of Arizona, Tucson, AZ, United States, 7Electrical and Computer Engineering, The University of Arizona, Tucson, AZ, United States, 8Biomedical Engineering, The University of Arizona, Tucson, AZ, United States
Synopsis
Keywords: Machine Learning/Artificial Intelligence, Image Reconstruction
Unrolled networks with data consistency layers have been shown to be effective in reconstructing MRI data. Radial turbo spin echo sequences enable acquisition of multi-contrast k-space data, which can be used to generate multi-contrast images at different echo times together with a co-registered T2 map. In this work, we will show that the cascading unrolled network architecture is effective in reconstructing images from radial turbo spin echo data. In order to do this, data consistency layers must be implemented to be able to combine data from multi-coil acquisitions and from multiple echo times.Introduction
Radial MRI is naturally robust to motion-induced and under-sampling artifacts and can accelerate data acquisition for parameter mapping applications1. In particular, the Radial Turbo-Spin-Echo (RADTSE)2 sequence acquires data that can provide co-registered images with different contrasts at various echo times (TE), which can be used to generate a co-registered T2 map. A recent work has shown the efficacy of a patch-based deep learning model to reconstruct RADTSE data faster than iterative compressed sensing techniques3. In this work, we utilize a novel deep learning approach to reconstruct RADTSE data with a cascading architecture of unrolled networks4. The approach alternates between convolutional neural network (CNN) blocks for artifact suppression and data consistency (DC) blocks to promote fidelity to acquired k-space data.Methods
RADTSE abdominal data sets from 18 consenting subjects were acquired on a 3T Siemens Skyra scanner with echo spacing=8.1ms, echo train length (ETL)=32, TR=2000ms, and 10 lines per TE with 256 readout points per line. Each subject was scanned twice using two variants of the RADTSE technique5, a constant flip angle (CFA) and a variable flip angle (VFA), resulting in 36 total data sets, each with 28 axial slices of the abdomen.The data were used for two separate tasks. The first is to reconstruct composite images, a term used to refer to the image reconstructed with data from all TEs jointly. For this first task, an unrolled network was designed to predict highly sampled composite images from under-sampled data. For the first task, data from 3, 4, or 5 of the 10 TRs (96, 128, or 160 of the 320 radial lines) were kept while the rest were zero-filled, resulting in a retrospectively under-sampled data set. Composite images were generated from these data sets using a non-uniform fast Fourier transform (NUFFT)6 reconstruction. The retrospectively under-sampled data sets were used as inputs to the first network. The data set with 320 radial views was used as the target.
The second task is to reconstruct TE images and a T2 map. For this task, acquiring fully sampled reference data requires prohibitively long acquisitions. As an alternative, the 320 radial line data set (10 per TE) was reconstructed using the locally low rank (LLR)7 compressed sensing (CS) algorithm to use as targets for the network. LLR is used in this task because data at each TE is highly under-sampled compared to the composite image (as illustrated in Figure 1). The data were also reconstructed using NUFFT to use as inputs for the network. LLR produces high-quality TE images but is computationally inefficient and is therefore not practical in a routine clinical setting. LLR reconstructs an image compressed to the first 4 principal components of the temporal dimension using a singular value decomposition compression matrix. The unrolled network is developed to be applied on compressed images to save memory during training.
Formulation of an unrolled network poses a challenge in this case because projecting from image space to data space and vice-versa requires a concatenation of distinct NUFFTs for each TE, a coil combination8 operator, and projection to the temporal subspace. The DC step occurs in k-space, so this back-and-forth projection must be done for every DC block.
The network for composite images iterates between 5 CNN blocks and 5 DC blocks, with each CNN block consisting of a 5-layer residual CNN. Each CNN block has its own unique parameters and training was performed end-to-end. The spatio-temporal reconstruction network was similarly constructed but the CNN blocks had identical parameters in order to reduce the number of trainable parameters due to memory limitations. The architecture for the composite and spatio-temporal networks can be seen in Figures 2 and 3. These networks were implemented using the Merlin9 package in Pytorch10.
Results and Discussion
The results from the composite network are shown in Figure 4. The results show that the network significantly reduces radial under-sampling artifacts, which can obscure pathology. Reduction of under-sampling artifacts is observed consistently across different under-sampling ratios. The results from the spatio-temporal network are provided in Figure 5, where 4 of the 32 TE images are shown. The network removes the under-sampling artifacts present in NUFFT images and yields image quality similar to what is provided by LLR, at a fraction of the reconstruction time (approximately 45 minutes/slice for LLR compared to 7 seconds/slice for the proposed network). Figure 5 also shows T2 maps generated using pixel-wise fingerprint matching11. It can be observed that anatomical features are well delineated in T2 maps obtained using the proposed network and LLR.Conclusion
This study presents two cascading unrolled network architectures to be used on multi-coil multi-contrast radial data sets. The first architecture enables predicting highly quality composite images from under-sampled data. The second network enables reconstruction of multi-contrast TE images, as well as a T2 map, much more efficiently than commonly used iterative CS methods.Acknowledgements
We would like to acknowledge grant support from the National Institutes of Health (CA245920 and EB031894), Arizona Biomedical Research Centre (CTR056039), and the Technology and Research Initiative Fund (TRIF) Improving Health Initiative.
References
- Altbach M, Trouard T, Gmitro A. Radial MRI techniques for obtaining motion-insensitive high-resolution images with variable contrast. IEEE (2002), 125–128.
- Altbach M, Outwater E, Trouard T, et al. Radial fast spin-echo method for T2-weighted imaging and T2 mapping of the liver. Journal of Magnetic Resonance Imaging 16, 2 (2002), JMRI, 179–189.
- Fu Z, Mandava S, Keerthivasan MB, Li Z, Johnson K, Martin DR, Altbach MI, Bilgin A. A multi-scale residual network for accelerated radial MR parameter mapping. Magnetic Resonance Imaging 73, (2020), 152-162.
- Schlemper J, Caballero J, Hajnal J, et al. A deep cascade of convolutional neural networks for MR image reconstruction. In International conference on information processing in medical imaging (2017), Springer, 647–658.
- Keerthivasan M, Galons J P, Johnson K, et al. Abdominal T2-weighted imaging and T2 mapping using a variable flip angle radial turbo spin-echo technique. Journal of magnetic resonance imaging (2021), JMRI, 55(1), 289–300.
- Fessler J A. http://web.eecs.umich.edu/ fessler/irt.
- Huang C, Graff C, Clarkson E, et al. T2 mapping from highly under-sampled data by reconstruction of principal component coefficient maps using compressed sensing. Magnetic resonance in medicine (2012), 67(5), 1355–1366.
- Uecker M, Lai P, Murphy M J, et al. Espirit—an eigenvalue approach to autocalibrating parallel MRI: where sense meets grappa. Magnetic resonance in medicine 71, 3 (2014), 990–1001.
- Hammernik K, K ̈ustner T. Machine enhanced reconstruction learning and interpretation networks (MERLIN). Annual Meeting of the ISMRM (2022), ISMRM.
- Paszke A, Gross S, Massa F, et al. Pytorch: An imperative style, high-performance deep learning library. In Advances in Neural Information Processing Systems 32. Curran Associates, Inc. (2019), pp. 8024–8035.
- Huang C, Altbach M, El Fakhri G. Pattern recognition for rapid T2 mapping with stimulated echo compensation. Magnetic resonance imaging (2014), 32(7), 969–974.
Figures
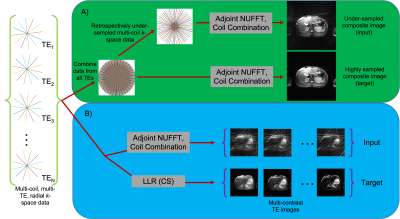
Figure 1: Multi-coil, multi-TE radial k-space data is acquired and used in two processing pipelines. A) Input and target data for the composite network are created by combining data from all TEs into a single data set. Highly sampled data is used as target. Under-sampled data at various under-sampling ratios is used as input. Composite images from these data sets are shown. B) For the spatio-temporal network, the temporal dimension of the acquired training data is preserved, and the corresponding NUFFT and LLR reconstructions used as input and target, respectively, are shown.
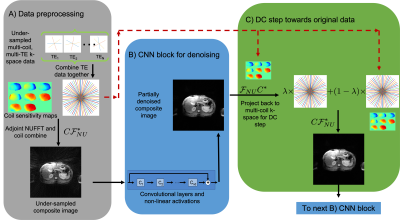
Figure 2: Blocks of the composite network: A) To preprocess the data, combine data from individual TEs into single data set, then apply an adjoint NUFFT and coil combine. B) CNN blocks use convolutions and activations to denoise the image. C) DC blocks project the image back into k-space and take a convex combination of the current k-space and the originally acquired data, then project back into image space to feed into the next CNN block. CNN and DC blocks are repeated a prescribed number of times.
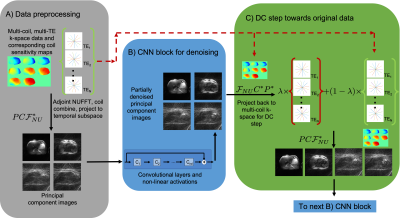
Figure 3: Blocks of the spatio-temporal network: A) To preprocess the data, apply adjoint NUFFTs per TE, combine coils, and project into a temporal subspace to form principal component (PC) images. B) CNN blocks use convolutions and activations to denoise the PC images. C) DC blocks project the image back to the original space and take a convex combination with the original k-space data. Then project back into PC image space to feed into the next CNN block. CNN and DC blocks are repeated a prescribed number of times.
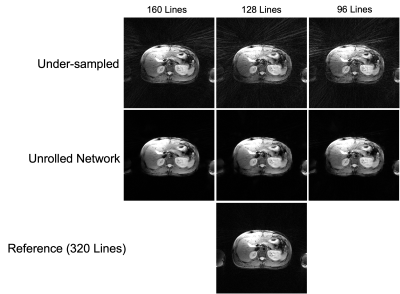
Figure 4: Results of the composite network, where the inputs (top), outputs (middle), and target (bottom) can be seen. Three different under-sampling ratios are shown in the columns, corresponding to data acquired in 5 (160 lines), 4 (128 lines), and 3 (96 lines) TRs, respectively.

Figure 5: Select TE images to show the results of the spatio-temporal network. TEs 5, 10, 15, and 25 are shown, corresponding to 40.5, 81, 121.5, and 202.5ms, respectively. Data for each TE consists of 10 radial lines. On the top row, images are generated with an adjoint NUFFT and coil combination. In the middle, images are outputs of the unrolled network. Images in the bottom row are created with LLR. Signal decay per pixel is fitted into T2 parameter maps, shown on the right column.
DOI: https://doi.org/10.58530/2023/0958