0942
Deep learning-based non-rigid motion-corrected reconstruction for whole-heart multi-dimensional joint T1/T2 imaging1School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 2School of Biomedical Engineering, ShanghaiTech University, Shanghai, China, 3MR Research Collaborations, Siemens Healthcare Limited, Camberley, United Kingdom
Synopsis
Keywords: Machine Learning/Artificial Intelligence, Motion Correction
Myocardial T1 and T2 mapping play an important role in the assessment of cardiovascular disease. 3D whole-heart joint T1/T2 water/fat mapping approaches have been recently proposed, however they require long reconstruction times. Recently a Machine learning based reconstruction was proposed for joint motion correction and motion corrected image reconstruction of undersampled free-breathing single contrast 3D coronary MR angiography. Here, we extend this approach for non-rigid motion-corrected reconstructions for multi-contrast data for joint T1/T2 mapping. The proposed approach achieves good agreement with reference techniques and comparable image quality to state-of-the-art methods albeit in 1200 times shorter reconstruction times.Introduction
Myocardial T1 and T2 mapping play an important role in the assessment of cardiovascular disease (CVD)1. Recently, a free-breathing high resolution, motion compensated 3D joint T1/T2 water/fat sequence has been proposed and validated in phantom and healthy subjects2. To obtain the 3D whole-heart T1 and T2 maps, a reconstruction of eight intermediate motion-compensated volumes are needed, which is very time consuming. In this work, we propose to extend the previously introduced end-to-end deep learning (DL) technique initially applied to nonrigid motion-corrected reconstruction of undersampled free-breathing whole-heart coronary MRA3 for multi-parametric isotropic joint T1/T2 data. This allows a concurrent estimation of the motion fields and motion corrected reconstructions from the undersampled data, while increasing the speed and maintaining the quality of the reconstructionsMethods
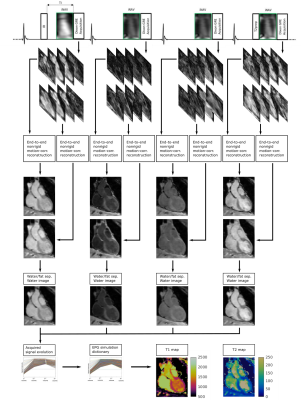
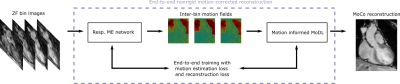
The proposed pipeline is shown in Figures 1 and 2 for acquisition and reconstruction, respectively. The joint T1/T2 water/fat mapping research sequence2 uses a 4x undersampled variable density cartesian trajectory3 and acquires four interleaved, ECG-triggered spoiled gradient-echo volumes with 2-point bipolar Dixon encoding. Prior to the first and fourth volume an inversion recovery and T2 preparation pulse is used, whereas no preparation is used prior the second and third interleaves (Fig. 1).The proposed DL-based reconstruction for multi-contrast undersampled free-breathing whole-heart data is extended from the end-to-end DL technique for nonrigid motion-corrected reconstruction of single contrast data (MoCo-MoDL)4. MoCo-MoDL consists of a diffeomorphic respiratory motion estimation network in combination with a motion-informed model-based deep learning reconstruction network. We adapted the network in order to process all eight contrasts, i.e. four interleaves and respective dual-echo signals (Fig. 1). We trained a network for each of the eight contrasts using an ADAM optimizer with an initial learning rate of 2x10-4, which was reduced by half every 2000 iterations. The network was trained for 100 epochs with a training/testing split of 52/6. The same network architecture and hyper-parameters was used for each contrast.
After reconstruction, a water-fat separation algorithm is applied and a patient specific dictionary of pre-calculated T1/T2 combinations is matched against the measured water image signal evolution, resulting in the final T1/T2 maps2. The precomputed T1/T2 combinations in the dictionary were simulated using the EPG formalism6 and are in the ranges T1: 60:10:2000 ms, T2: 4:2:100, 100:5:200, 200:10:300 ms.
Acquisitions were performed at 1.5T (MAGNETOM Aera, Siemens Healthcare, Erlangen, Germany) on 58 subjects (34 patients with suspected CVD and 24 healthy subjects). Main acquisition parameters were FA=8˚, TR = 6.67ms, TE1/TE2=2.38/4.76ms, 2 mm3 isotropic resolution and a subject specific mid-diastolic trigger-delay and acquisition window of ~100ms, resulting in a total scan time of nine minutes. The data was pre-processed for each contrast by computing a high quality refence image with a patch-based higher order low-rank (HD-PROST) reconstruction5. These high-quality respiratory bin images, together with undersampled respiratory bin images for all contrasts reconstructed using zero-filling, are inputted into the network.
Results
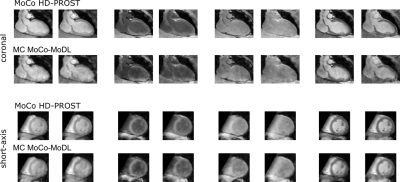
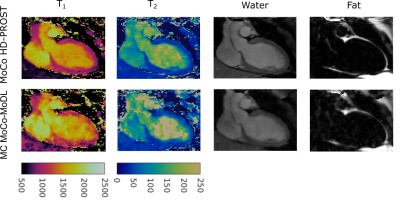
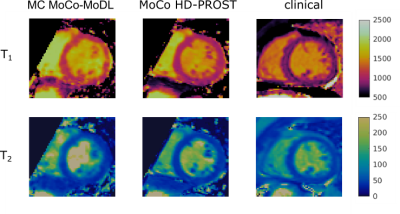
Reconstruction results for the proposed multicontrast MoCo-MoDL (MC MoCo-MoDL) are shown in Fig. 3 for all eight contrasts in comparison to state-of-the-art non-rigid motion-corrected HD-PROST(MoCo HD-PROST) reconstruction. Comparable visual image quality is achieved between the proposed approach and MoCo HD-PROST. 3D T1/T2 maps for the proposed MC MoCo-MoDL reconstruction in comparison to MoCo HD-PROST are shown in Fig. 4 for a representative subject. Mid-ventricular septum T1 and T2 values of 1171ms and 44ms, and 1021ms and 43ms were measured for MC MoCo-MoDL and MoCo HD-PROST, which were in good agreement with reference clinical 2D MOLLI and T2 prepared bSSFP maps (840ms and 53ms respectively) (Fig. 5). Comparable visual image quality is achieved between the proposed approach and MoCo HD-PROST for T2 mapping however remaining artefacts are observed with MC MoCo-MoDL for T1 mapping. Reconstruction time per contrast was of 3s for the proposed MC MoCo-MoDL and of 2 hours for MoCo HD-PROST.Discussion
This work is a proof of concept study and comprehensive tuning of the hyper parameters has not been performed yet. We believe that tuning the hyper parameters in the proposed network overall and per contrast can further improve the reconstruction quality, especially for T1 mapping. Furthermore, a joint reconstruction of all contrasts with shared network parameters could improve the reconstruction quality further, and this will be investigated in future work.Conclusion
We have proposed an end-to-end deep learning approach for non-rigid motion-corrected reconstructions for multi-dimensional 3D whole-heart joint T1/T2 mapping. The proposed approach achieves similar reconstruction quality as the non-rigid HD-PROST reconstruction used as reference, albeit with significantly shorter reconstruction times (1200 times faster). The reconstruction time is reduced to three seconds per contrast, which may make the approach promising for clinical application.Acknowledgements
This work was supported by the following grants: (1) EPSRC P/V044087/1; (2) BHF programme grant RG/20/1/34802, (3) Wellcome/EPSRC Centre for Medical Engineering (WT 203148/Z/16/Z), (4) Millennium Institute for Intelligent Healthcare Engineering ICN2021_004, (5) FONDECYT 1210637 and 1210638, (6) IMPACT, Center of Interventional Medicine for Precision and Advanced Cellular Therapy, ANID FB210024.References
1. Messroghli, D.R., Moon, J.C., Ferreira, V.M. et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J Cardiovasc Magn Reson 2017; 19, 75.
2. Milotta, G, Bustin, A, Jaubert, O, Neji, R, Prieto, C, Botnar, RM. 3D whole-heart isotropic-resolution motion-compensated joint T1/T2 mapping and water/fat imaging. Magn Reson Med. 2020; 84: 3009– 3026.
3. Qi, H, Hajhosseiny, R, Cruz, G, et al. End-to-end deep learning nonrigid motion-corrected reconstruction for highly accelerated free-breathing coronary MRA. Magn Reson Med. 2021; 86: 1983– 1996.
4. Prieto C, Doneva M, Usman M, et al. Highly efficient respiratory motion compensated free-breathing coronary MRA using golden-step Cartesian acquisition. J Magn Reson Imaging. 2015; 41: 738-746.
5. Bustin A, Ginami G, Cruz G, et al. Five-minute whole-heart coronary MRA with sub-millimeter isotropic resolution, 100% respiratory scan efficiency, and 3D-PROST reconstruction. Magn Reson Med. 2019; 81: 102-115.
6. Weigel, M. Extended phase graphs: Dephasing, RF pulses, and echoes - pure and simple. J. Magn. Reson. Imaging 2015; 41: 266-295.
Figures