0936
Phase-Unwrapping based on Graph-Cuts for Low-venc Phase-Contrast Cine MRI
Johan Berglund1
1Dept. of Surgical Sciences, Uppsala University, Uppsala, Sweden
1Dept. of Surgical Sciences, Uppsala University, Uppsala, Sweden
Synopsis
Keywords: Flow, Velocity & Flow
The use of low velocity encoding (venc) improves the velocity-to-noise ratio in phase-contrast MRI, but increases the risk of phase wrapping. A novel phase-unwrapping technique based on graph-cuts was evaluated in five cardiac patients with high-, medium-, and low-venc acquisitions. The proposed method reduced the stroke volume error compared to a LaPlacian based reference method. In retrospectively wrapped data, the proposed method demonstrated excellent unwrapping results in most cases, even for venc as low as 25 cm/s. The graph-cut based method appears promising for low-venc acquisitions combined with phase-unwrapping.Introduction
Phase-contrast cine MRI is commonly used in cardiovascular examinations for the assessment of flow in the great vessels. Since the velocity encoding range ± venc is mapped onto the phase-contrast image, velocities outside this range will be subject to phase wrapping. Nonetheless, it is desirable to keep venc low since it is inversely proportional to the velocity-to-noise ratio1. Therefore, venc is typically set to the expected maximum velocity plus a safety margin for each patient. This procedure adds complexity to the examination and requires retakes if the maximum velocity is underestimated. As an alternative, acquisition with venc below the maximum velocity may be combined with phase-unwrapping, provided reliable algorithms are available2. In this work, we explore phase-unwrapping using graph-cuts, which have previously been successfully applied for fat/water separation3.Methods
We developed a novel, fully automatic phase-unwrapping algorithm which imposes phase smoothness by attempting to minimize the objective function$$E(\theta) = \sum_{p, q}w_{p,q}(\theta_p-\theta_q)^2,$$
where $$$p,q$$$ are all spatial and temporal neighbor voxel pairs, $$$\theta$$$ is the phase, and $$$w$$$ are weights. The solution in each voxel is constrained to the measured phase plus integer multiples of $$$2\pi$$$. This large optimization problem is solved by iteratively performing graph-cuts, corresponding to a so-called $$$2\pi$$$ jump moves, until convergence4.
2D phase-contrast cine images were acquired from five cardiac patients, who gave written informed consent. For each patient, three different planes were acquired targeting the pulmonary artery, the sinotubular junction, and the ascending/descending aorta. In addition to ordinary imaging with venc=150–330 cm/s, the acquisitions were repeated with venc=100 cm/s (“mid venc”) and 50 cm/s (“low venc”). The study was approved by the national ethics review authority.
In addition, the high venc acquisitions were retrospectively phase wrapped to obtain a "retrospective venc" equal to 100, 50, and 25 cm/s.
All the wrapped images were unwrapped using the proposed graph-cut method and a LaPlacian-based method for reference5, both of which were implemented in the Python programming language. The stroke volume in each vessel was assessed by integrating the velocity over the cardiac cycle and vessel area, based on semi-automatic segmentation.
Results
No phase wrapping was seen in the high-venc acquisitions. Most mid-venc acquisitions showed only limited phase wrapping, while all low-venc acquisitions demonstrated severe phase wrapping. Box plots of the stroke volume error for mid- and low-venc compared to high-venc acquisition are shown in Fig. 1. The average stroke volume error for No unwrapping / LaPlace / Graph-Cuts was -11.0 / -7.9 / -6.0 mL for venc=100 cm/s and -61.4 / -11.8 / -7.4 mL for venc=50 cm/s.Representative images and flow measurements from retrospectively wrapped data are shown in Fig. 2. For the retrospectively wrapped data, the original image was used as ground truth to assess the amount of phase wrapped voxels within the segmented vessel, with and without unwrapping (box plots shown in Fig. 3). The average amount of wrapped voxels for No unwrapping / LaPlace / Graph-Cuts was 1.2 / 0.3 / 0.0 % for retrospective venc=100 cm/s, 12.4 / 1.2 / 0.5 % for retrospective venc=50 cm/s, and 25.3 / 7.9 / 3.6 % for retrospective venc=25 cm/s.
Discussion
In most cases, the unwrapping performance of the proposed method was superior to the reference method. The processing time was about 30 seconds per dataset. Implementation in a compiled language is expected to result in processing times acceptable for use in clinical practice. The consistent use of a medium to low venc combined with phase-unwrapping can simplify cardiac examinations and reduce the number of retakes. Outliers in Figs. 1 & 3 correspond to a subject with aortic valve stenosis and a peak velocity of 290 cm/s in the aorta. Since such challenging cases will appear in the clinic, it will still be required to screen phase-contrast images for wrapping and possibly re-acquire with a higher venc.Conclusion
The proposed method appears promising to enable the consistent use of a medium to low venc in clinical practice while avoiding errors due to phase wrapping.Acknowledgements
No acknowledgement found.References
- Pelc NJ, Herfkens RJ, Shimakawa A, Enzmann DR. Phase contrast cine magnetic resonance imaging. Magn. Reson. Q. 1991; 7:229–254.
- Axel L, Morton D. Correction of phase wrapping in magnetic resonance imaging. Med. Phys. 1989; 16:284–287.
- Hernando D, Kellman P, Haldar JP, Liang ZP. Robust water/fat separation in the presence of large field inhomogeneities using a graph cut algorithm. Magn. Reson. Med. 2010; 63:79– 90.
- Bioucas-Dias JM, Valadao G. Phase unwrapping via graph cuts. IEEE Trans. Image Process. 2007; 16:698–709.
- Loecher M, Schrauben E, Johnson KM, Wieben O. Phase unwrapping in 4D MR flow with a 4D single-step laplacian algorithm. J. Magn. Reson. Imaging 2016; 43:833–842.
Figures
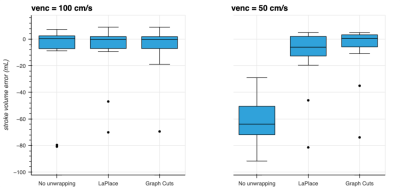
Figure 1: Stroke volume error for medium- and low-venc acquisitions using the stroke volume assessed from the high venc acquisition as ground truth. For medium venc (100 cm/s), the error was quite small even without phase-unwrapping. The use of low venc (50 cm/s) resulted in large stroke volume errors due to phase wrapping. These could be largely counteracted by the LaPlace unwrapping method, while the graph-cut based method resulted in even smaller errors on average.
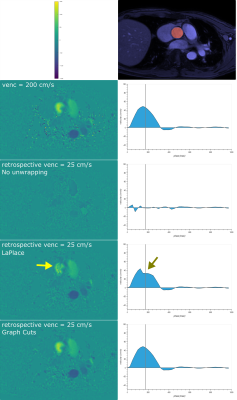
Figure 2: Semi-automatic segmentation (top right) was used to assess the flow curves in the right column. The left column shows phase-contrast images without wrapping (second row) and with retrospective wrapping corresponding to venc=25 cm/s (rows 3–5). The LaPlace based unwrapping technique (fourth row) presented some residual wrapping which distorted the flow curve (arrows), while the proposed graph-cut based technique (last row) demonstrated perfect unwrapping results in this specific case.
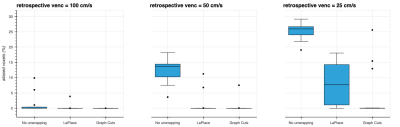
Figure 3: Percentage of aliased voxels within the segmented target vessel. Retrospective wrapping corresponding to venc equal to 100 and 50 cm/s introduced wrapped voxels, which could be successfully restored by both unwrapping methods in most cases. Retrospective venc equal to 25 cm/s resulted in large amounts of wrapped voxels, which could only be partially restored by the LaPlace based method. The proposed method demonstrated perfect unwrapping, except for a few outlier cases.
DOI: https://doi.org/10.58530/2023/0936