0926
Respiration-resolved 5D flow MRI: Impact of the number of respiratory states of blood flow quantification in congenital heart disease patients1Radiology, Northwestern University, Chicago, IL, United States, 2University of Lausanne (CHUV), Lausanne, Switzerland, 3Cardiology, Ann & Robert Lurie Children's Hospital, Chicago, IL, United States, 4Medical Imaging, Ann & Robert Lurie Children's Hospital, Chicago, IL, United States
Synopsis
Keywords: Flow, Velocity & Flow, congenital heart disease
We adapted our novel respiratory gating method to evaluate the impact of respiratory state (RS) resolution on respiratory driven flow measured by 5D flow MRI. We found that the impact of respiratory state resolution was both anatomy and vessel dependent. Caval veins and measurements in single ventricle disease patients were most impacted by the reduction of respiratory states. Shunt patients and pulmonary artery measurements were more robust to reduced RS and may be able to take advantage of the decreased acceleration associated with fewer RS. 5D flow MRI is well suited for this variable need as respiratory gating is retrospective.Introduction
Respiration has been shown to drive hemodynamic changes in healthy individuals1 and patients with congenital heart disease (CHD).2 Yet, conventional flow methods including 2D phase contrast and 4D flow MRI are often limited to acquisition during a single respiration state (breath-hold, navigator-gated). 5D flow MRI3 is a new free-running, self-gated technique that enables 3D velocity quantification over both cardiac and respiratory cycles. However, the original respiratory gating method (Fig1a) did not reflect the physiology of respiration. Additionally, while four respiratory states (RS) were typically reconstructed, it is unclear if this respiratory resolution is optimal. 5D flow uses highly accelerated, radial sampling combined with compressed sensing (CS) reconstruction. Reduction of RS may decrease acceleration, enabling improved image quality or shorter scan time. We aimed to assess the impact of RS resolution on respiratory driven flow measurements in a novel physiology driven gating method.Methods
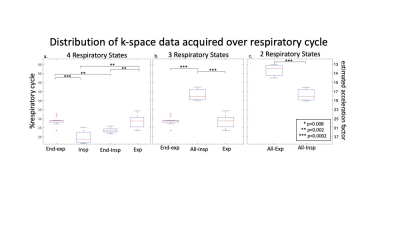
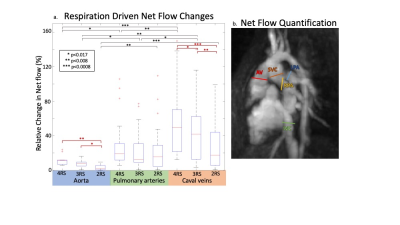
11 patients with CHD (15.6±11.7yrs, M/F: 5/6) undergoing a clinically indicated CMR with ferumoxytol were consented for an 8.5-minute 5D flow add-on (res: 1.5-2.5mm isotropic, cardiac res: 40ms). Superior-inferior readouts were used to extract cardiac and respiratory signals for retrospective gating. Scans were reconstructed three times: with 2, 3, or 4 RS. The physiology driven gating method sorted readouts on a per-breath basis, assigning the maximum and minimum 25% of signal to end-expiration and end-inspiration respectively. To define 4 RS, the remaining points were assigned to expiration and inspiration based on slope (Fig1b). To create 3 RS, inspiration and end-inspiration were combined (Fig1c). For 2 RS, end-expiration and expiration were also combined (Fig1d). These reductions aimed to decrease CS acceleration. The acceleration was patient-specific, based on respiratory gating (Fig2). Net flow was measured in the ascending aorta (Ao), main pulmonary artery (MPA), left and right pulmonary arteries (LPA&RPA), and inferior and superior vena cava (IVC&SVC). Range of net flow over all RS, normalized to the mean over respiration, defined respiratory driven flow changes. Median respiratory resolved flow curves were normalized to end-expiration. The cohort was stratified into two subgroups: single ventricle disease (SVD, n=5) and shunt physiology (n=5). One patient fit neither group.Results
Respiratory gating and CS acceleration: Data acquired during expiration and end-expiration covered a significantly larger fraction of the respiratory cycle than inspiration (28% & 29% vs 20%, p<0.01) and end-inspiration (28% & 29% vs 23%, p<0.01). With reduced RS resolution, the necessary CS acceleration decreased (Fig2).Respiratory driven net flow changes: Reduced RS resolution decreased respiratory driven flow changes measured in the aorta, pulmonary arteries (PAs, MPA, LPA&RPA), and caval veins (IVC&SVC). Flow changes were significantly larger in 4 RS images compared to 3 and 2 RS images in the aorta (12% vs 8% & 3%, p<0.017) and caval veins (59% vs 46% & 28%, p<0.017). Caval veins had significantly larger flow changes than the PAs in 4 RS (28% vs 59%, p<0.017) and 3 RS (22% vs 46%, p<0.017) images, but not in 2 RS images. The aorta had significantly smaller respiratory driven changes than the PAs and caval veins in all images (Fig3).
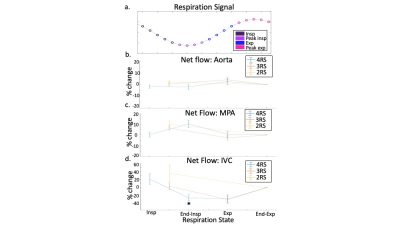
Respiratory resolved flow curves: Timing of changes in flow over respiration were generally conserved with reduced RS (Fig4). However, in the IVC, the relative change in flow during inspiration in 4 RS images was significantly less than in 2 RS images (Fig4c, p<0.01).
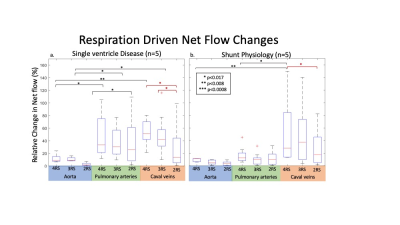
Sub-group analysis: In 4 RS images, SVD patients had increased flow changes in PAs and caval veins compared to the aorta (Fig5). In shunt patients, caval veins had increased flow changes compared to the aorta and PAs. Only SVD patients had a substantial decrease in respiratory driven flow in caval veins from 3 to 2 RS. Additionally, inter-vessel group differences became insignificant for shunt patients when using <4 RS whereas differences were preserved using 3 RS in SVD patients.
Discussion
Overall, we found that the impact of respiratory resolution was vessel dependent. Respiratory driven flow decreased with RS resolution in the aorta and caval veins, but not in the PAs. This may be due to similar flow changes during end-inspiration and inspiration, leading to little information loss when combined. Yet, differences between PAs and caval veins were diminished in the 2 RS images. Due to retrospective self-gating, 5D flow is well-suited to the need for variable RS resolution, allowing reduced CS acceleration in cases where 2 or 3 RS is sufficient. Additionally, in the IVC, end-inspiration and inspiration had different effects on flow. This information was lost in both the 2 and 3 RS images, suggesting 4 RS may be necessary to resolve respiratory driven flow in caval veins. We also showed that SVD anatomy may benefit more from additional RS than shunt anatomy. There was significant change in the caval veins with each respiratory state reduced only in the SVD group. Future studies should include additional CHD types and healthy volunteers to optimize respiratory resolution for each subject group.Conclusion
We found that impact of respiratory resolution on flow was both anatomical subgroup and vessel dependent. In the caval veins and aorta, but not the PAs, reduction in RS decreased measured respiratory driven flow. Caval vein flow changes diminished with each combined RS in the SVD group only.Acknowledgements
No acknowledgement found.References
1. Körperich, H., Barth, P., Gieseke, J., Müller, K., Burchert, W., Esdorn, H., ... & Laser, K. T. (2015). Impact of respiration on stroke volumes in paediatric controls and in patients after Fontan procedure assessed by MR real-time phase-velocity mapping. European Heart Journal-Cardiovascular Imaging, 16(2), 198-209.
2. Wei, Z., Whitehead, K. K., Khiabani, R. H., Tree, M., Tang, E., Paridon, S. M., ... & Yoganathan, A. P. (2016). Respiratory effects on Fontan circulation during rest and exercise using real-time cardiac magnetic resonance imaging. The Annals of thoracic surgery, 101(5), 1818-1825.
3. Ma, L. E., Yerly, J., Piccini, D., Di Sopra, L., Roy, C. W., Carr, J. C., ... & Markl, M. (2020). 5D flow MRI: a fully self-gated, free-running framework for cardiac and respiratory motion–resolved 3D hemodynamics. Radiology: Cardiothoracic Imaging, 2(6).
Figures
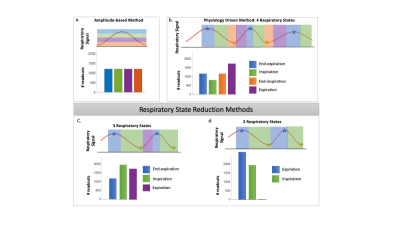
Figure 1. Respiratory gating methods overview. Initial implementation of 5D flow MRI used an amplitude-based method to determine respiratory states (a, RS). We have proposed a novel approach, defining a gating method based on physiologic respiratory states (b). In this abstract, we propose two methods to reduce the number of respiratory states (c-d). The number of readouts per respiratory state is patient specific as determined by the gating method.