0900
Highly Accelerated 3D EPI CEST Imaging using Unevenly Segmented RF Irradiation with Temporal Random Walk Sampling Pattern in a Brain Tumor Patient
Hahnsung Kim1,2, Suhyung Park3,4, Ranliang Hu2, Kimberly B Hoang5, and Phillip Zhe Sun1,2
1Emory National Primate Research Center, Emory University, Atlanta, GA, United States, 2Department of Radiology and Imaging Sciences, Emory University School of Medicine, Atlanta, GA, United States, 33Department of Computer Engineering, Chonnam National University, Gwangju, Korea, Republic of, 4Department of ICT Convergence System Engineering, Chonnam National University, Gwangju, Korea, Republic of, 5Department of Neurosurgery, Emory University School of Medicine, Atlanta, GA, United States
1Emory National Primate Research Center, Emory University, Atlanta, GA, United States, 2Department of Radiology and Imaging Sciences, Emory University School of Medicine, Atlanta, GA, United States, 33Department of Computer Engineering, Chonnam National University, Gwangju, Korea, Republic of, 4Department of ICT Convergence System Engineering, Chonnam National University, Gwangju, Korea, Republic of, 5Department of Neurosurgery, Emory University School of Medicine, Atlanta, GA, United States
Synopsis
Keywords: CEST & MT, CEST & MT
We proposed a new 3D EPI CEST imaging integrated with unevenly segmented RF irradiation configuration to reduce T1w relaxation-induced signal modulation, thereby allowing for a reliable CEST effect over the whole volume. In addition, a temporal random walk with variable density (VD) CAIPI undersampling is incorporated into segmented 3D EPI for optimized random encoding in CEST imaging. The proposed pulse sequence and reconstruction framework were validated on the phantom and tumor patient.INTRODUCTION:
3D CEST MRI is often implemented with a long RF irradiation followed by a rapid image readout sequence such as EPI to cover the whole volume of the object [1-3]. However, increasing the spatial and spectral coverage is challenging without extending the scan time or losing some of the CEST effects. An unevenly segmented RF irradiation module, in which a long primary RF pulse generates the steady-state CEST effect with repetitive short secondary RF irradiation to maintain the CEST effect, has been developed for 2D multi-slice CEST imaging [4]. We propose a new 3D EPI CEST imaging integrated with unevenly segmented RF irradiation configuration to reduce T1w relaxation-induced signal modulation, thereby allowing for a reliable CEST effect over the whole volume. In addition, a temporal random walk with variable density (VD) CAIPI undersampling is incorporated into segmented 3D EPI for optimized random encoding in CEST imaging. Numerical simulations, phantom studies, and tumor patient studies were performed to validate optimal imaging protocols.METHODS:
Pulse Sequence Design: Fig. 1 shows the proposed 3D CEST imaging with unevenly segmented RF irradiation configuration. Following a relaxation delay, a long primary continuous wave RF saturation is applied to generate the CEST effect. Repetitive short RF saturation pulses are inserted between partition acquisition to maintain CEST contrast over the volumetric imaging. A rapid segmented 3D EPI readout accommodates as many ky phase encoding steps as possible in each repetition time.Reconstruction: Time-wise random blips allow uniform encoding in the spatial domain while random encoding along the temporal direction. SENSE-based spatiotemporal reconstruction was jointly imposed with multiple temporal priors (low rank and sparsity) (Fig. 1b) [5].
Numerical Simulation: Two-pool Bloch-McConnell simulations were performed to investigate the effect of imaging parameters on the SNR of CEST and imaging time efficiency. The labile proton ratio and exchange rate were assumed to be 0.1% and 100 s-1 at 3.5 ppm. We assumed Ts1/Ts2/Td = 1.5/0.3/1.5 sec, B1 = 1.0 uT and 24 excitations with centric reordering. Peak PSF amplitude and effective volume acquisition time were measured with varying FAs and segments.
Experimental Studies: All data were obtained at a 3T Siemens whole-body MAGNETOM Prisma scanner (Siemens Healthineers). Informed written consent was obtained following IRB-approved protocol.
Phantom studies: Phantom data in the sagittal orientation were acquired. The mixture of 1.5% agarose and 100 mM l-carnosine was doped with MnCl2 (15μM, 30μM). Two vials were inserted into a 500 ml container with 1.5% agarose. The MRI parameters were: B1 = 1.0 uT, offset frequencies from -5 to 5 ppm with increments of 0.125 ppm, Ts1/Ts2 = 1.5/0.3 sec, Td = 1.5 sec, FOV = 140x108x80 mm3, 2 mm isotropic spatial resolution, and the number of partition = 40. CEST contrast was measured with varying FAs from 10° to 80° with a 10° increment. We compared 3D CEST images with/without short secondary RF in axial-oriented images (y-z planes).
Volunteer studies: Two sets of 3D brain images with/without secondary RF saturation (Ts1/Ts2/Td =1.5/0.3/1.5 sec) were acquired in the axial orientation. B1 = 1.0 uT, offset frequencies from -5 to 5 ppm with increments of 0.125 ppm, FOV = 220x220x80 mm3, 2 mm isotropic spatial resolution, number of partition = 40, 6 segments, 6-fold acceleration, TR = 35 ms and water excitation RF pulse with FA = 20°. Total imaging times were 5 min 14 sec (without Ts2) and 6 min 28 sec (with Ts2), respectively.
Tumor patient studies: Two sets of the proposed 3D EPI CEST images with different B1 (1.0 and 2.0 uT) were acquired. For high-resolution anatomical imaging, 1 mm3 isotropic 3D MPRAGE imaging was used.
RESULTS/DISCUSSION:
Fig. 2 shows contour plots of relative SNR (rSNR) and imaging time efficiency (η). rSNR rises with increasing FA in a range of small FA (≤ 25°), while the amplitude of rSNR gradually falls with increasing FA. η increased with decreasing segments. Fig. 3 demonstrates the effect of FA on the CEST contrast of the proposed 3D CEST imaging. Compared to the conventional 3D CEST imaging without short saturation RF configuration, the proposed method showed high SNR CEST contrast in the range of small FA. In normal brain imaging, the proposed method showed clear CEST contrast without signal modulation artifact (Fig. 4). Due to the signal modulation along the z-direction, imaging blurring leads to losing CEST contrast in the conventional 3D CEST imaging. The proposed method clearly delineated tumor tissue with higher CEST sensitivity (Fig. 5).CONCLUSION:
Our work demonstrated 3D CEST imaging with unevenly segmented RF irradiation configuration reduces the loss of CEST contrast during volumetric acquisition. With a balance between SNR and imaging time, we found the optimal parameters of FA (near 20°) and ranges of segments (4~8). The proposed method integrated with the parallel imaging technique can be extended to clinical applications.Acknowledgements
This study was supported in part by grants from NIH/NINDS 2R01NS083654 (to Sun), and NRF/MSIT No. 2021R1C1C1013603 (to Park).References
[1] Akeby S, et al. Magn Reson Med 2019;82:1741-1752
[2] Sebastian M, et al., Magn Reson Med 2020;84:2469-2483
[3] Steffen G, et al., Magn Reson Med 2021;86:393-404
[4] Sun PZ et al., Magn Reson Med 2011;65:588-94
[5] Park S et al., In Proceedings of Annual Meeting of ISMRM, 2021, p0629
Figures
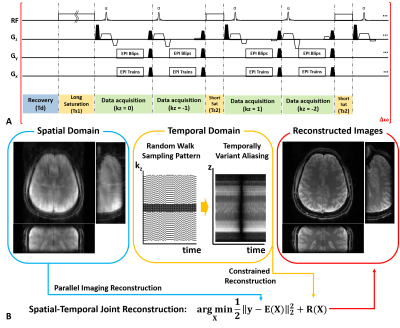
Figure
1. (A) A time diagram of the
proposed 3D EPI CEST imaging using unevenly segmented RF irradiation. (B) The
illustration of temporal random walk sampling pattern and reconstruction framework.
Note that proposed temporal random walk produces coherent aliasing in the
spatial domain, while yielding incoherent aliasing in the temporal domain.
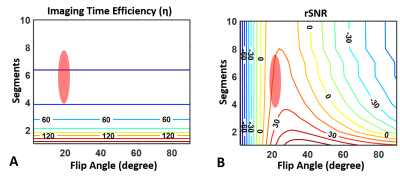
Figure
2. Contour plots of imaging time efficiency and relative SNR as a function
of FA and segments. Note that the optimal parameters of FA is near 20° and
ranges of segments is 4~8 with a balance between SNR and imaging time.
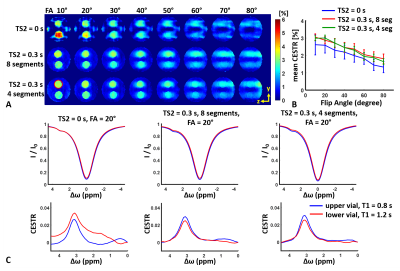
Figure
3. Fig 3.
(A) Axial oriented (y-z plane) CEST contrast images with varying FAs acquired
from the conventional 3D CEST imaging (1st row), the proposed method with 8
segments (2nd row), and 4 segments (3rd row). The Upper/ lower vial was doped 15
/ 30 μM MnCl2, respectively. (B) Mean CEST contrast as a function of FA. The red
circle indicates the ROI. (C) Z-spectrum and MTR asymmetric spectrum at FA =
20°. It is note that the conventional method overestimates CEST effects while
the proposed one
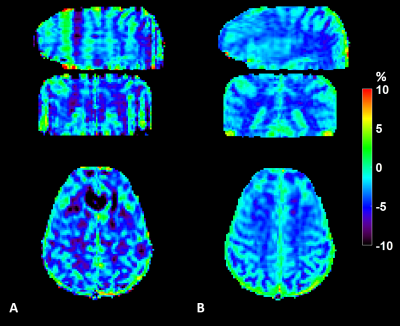
Figure
4. Comparison of 3D EPI CEST imaging (A) without short RF
saturation (B) with short RF saturation. Due to the signal modulation along the
z-direction, imaging blurring leads to losing CEST contrast in the conventional
3D CEST imaging (sagittal, coronal orientation)
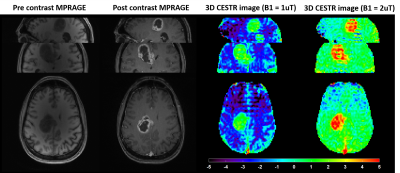
Figure
5. Comparison of the
proposed 3D EPI CEST imaging with different B1 power of 1 uT and 2 uT. Note that CESTR in tumor tissue
region and contralateral normal tissue region were 0.73±1.06%, -2.58±0.70%
with B1 = 1 uT, and 3.38±1.28%, 0.61±0.49% with B1 = 2 uT, respectively.
DOI: https://doi.org/10.58530/2023/0900