0892
Evaluation of deep learning high-resolution Dixon PET/MR attenuation correction using 16-channel head-neck and 32-channel head coils
Chunwei Ying1, Yasheng Chen2, Matthew R. Brier2, Shaney Flores1, Richard Laforest1, Tammie L. S. Benzinger1,2,3, and Hongyu An1,2
1Mallinckrodt Institute of Radiology, Washington University School of Medicine, St Louis, MO, United States, 2Department of Neurology, Washington University School of Medicine, St Louis, MO, United States, 3Department of Neurosurgery, Washington University School of Medicine, St Louis, MO, United States
1Mallinckrodt Institute of Radiology, Washington University School of Medicine, St Louis, MO, United States, 2Department of Neurology, Washington University School of Medicine, St Louis, MO, United States, 3Department of Neurosurgery, Washington University School of Medicine, St Louis, MO, United States
Synopsis
Keywords: PET/MR, Brain, attenuation correction
We evaluated the accuracy of a deep learning-based PET/MR attenuation correction (AC) method with vendor-provided high-resolution Dixon in- and opp-phase images as inputs (DL-HiRes). We found that the DL-HiRes AC method significantly outperformed the vendor-provided skull model AC method for both 16-channel head-neck coil and 32-channel head coil (p<0.001). Moreover, the DL-HiRes method had similar AC accuracy using different head coils.Introduction
Deep learning-based methods have demonstrated improved accuracy for PET attenuation correction (AC) compared to conventional methods(1-4). We recently developed a 3D patch-based residual UNet (ResUnet) structure to generate pseudo-CT (pCT) using various MRI scans for PET/MR AC(4). The DL-DIXON method provided excellent PET/MR AC accuracy using standard Dixon in- and opp-phase images as inputs(4).MR scanner upgrades, including software and hardware upgrades, are often introduced by the vendor. For example, a high-resolution (HiRes) Dixon scan was employed by the vendor to generate skull model μ-maps under the latest scanner software version (VE11P). In this skull model, the pre-saved skull bone is inserted through landmark matching. The skull model PET/MR AC is substantially better than the previously vendor-provided Dixon PET/MR AC(5). Moreover, a new 32-channel head coil was recently introduced to improve MR image quality for the Siemens Biograph mMR. Compared to the original 16-channel head-neck coil, images acquired using the 32-channel head coil have a higher degree of spatial signal variations. It remains unknown whether the MR coil may impact the performance of PET/MR AC. In this study, we trained a deep neural network using HiRes Dixon images (DL-HiRes). We evaluated the performance of DL-HiRes and the vendor-provided skull model using both the 16-channel head-neck and 32-channel head coils.
Methods
Tri-modality PET/MR (on a Siemens Biograph mMR 3T scanner) and PET/CT (on a Siemens Biograph mCT or Siemens Biograph Vision scanner) images were acquired from two groups of participants with IRB approval and informed consent. Group A consists of 24 participants (median [Interquartile range] age: 71.5 [68.8, 74.2], 14 female) with MR data acquired using the 16-channel head-neck coil. Group B consists of 24 participants (38.5 [28.8, 46.5], 19 female) with MR data acquired using the 32-channel head coil.Low-dose CT images were acquired at 120kVp from all participants. PET images were acquired using 18F-Florbetapir tracer for Group A, and 11C-PiB (N=3) or 18F-FDG (N=21) tracer for Group B. In- and opp-phase Dixon images were acquired using vendor-provided high-resolution Dixon AC scan (TE1/TE2/TR=1.28/2.51/4.14ms, FA=10°, voxel size=1.3x1.3x2mm, acquisition time=39sec).
The previously published DL-DIXON network trained using vendor-provided standard Dixon in- and opp-phase images (TE1/TE2/TR=1.23/2.46/3.6ms, FA=10°, voxel size=2.6x2.6x3.1mm, acquisition time=19sec) was used as the initial model for this study(4). The DL-HiRes networks were trained using in- and opp-phase high-resolution Dixon images as inputs. A network for 16-channel coil data was retrained using Group A, and a network for 32-channel coil data was retrained using Group B. We used three-fold cross-validation to evaluate the neural network performance: each time, 12 participants were used for training, 4 participants were used for validation, and 8 participants were used for testing.
The trained networks were applied to the high-resolution Dixon images to generate pCT images. CT and pCT images were converted to attenuation maps through a piecewise linear scaling(6). PET images were reconstructed using e7Tools with three attenuation maps: (1) gold standard CT-based μ-map. (2) DL-HiRes μ-map and (3) vendor-provided skull model μ-map derived using the high-resolution Dixon scan(7).
Results
The pCT mean absolute error (MAE) of DL-HiRes using the 16-channel coil is not significantly different from that using the 32-channel coil. (71.41±8.58HU vs 70.16±8.30HU, p=0.74; Figure 1). Figure 2 shows the CT μ-map, the DL-HiRes μ-map, and the skull model μ-map using the 16-channel coil (Figure 2A) and the 32-channel coil (Figure 2B) in representative participants.Two participants in Group A were excluded from PET evaluation due to excessive artifacts on the vendor-provided skull model μ-maps. Figure 3 illustrates the PET mean relative error (MRE) across the participants. As shown in Figure 4A, small PET MRE (within ±2.5%) was consistently observed using DL-HiRes. The PET mean relative absolute error (MRAE) of DL-HiRes was significantly smaller than that of skull model for both the 16-channel and 32-channel coils (p<0.001), while the same method performed similarly between the 16-channel and 32-channel coils (p>0.15) (Figure 4B). Using the 16-channel coil, 99.68% [99.35%, 99.84%] and 92.31% [90.59%, 94.76%] of brain voxels had PET MRAE less than 10% for DL-HiRes and skull model, respectively (Figure 5A,B). Using the 32-channel coil, 99.97% [99.89%, 100.00%] and 92.77% [89.54%, 95.94%] of brain voxels had PET MRAE less than 10% for DL-HiRes and skull model, respectively (Figure 5C,D).
Discussion
We demonstrated that the DL-HiRes method outperformed the skull model method using either the 16-channel or 32-channel coil. A recent consensus paper suggested using “voxelwise MRAE below 10% in at least 90% of the voxels in the brain mask” as a qualification criterion for PET/MR AC methods(5). The DL-HiRes method passed this criterion for all participants, while about 25% of participants failed this criterion in the skull model method using either the 16-channel or the 32-channel coils. Moreover, the DL-HiRes method had similar PET/MR AC accuracy using either the 16-channel or the 32-channel coil.Conclusion
Our results suggested that the DL-HiRes AC method is accurate, and has consistent performance when using different coils, supporting its use in PET/MR clinical studies.Acknowledgements
This study was supported by NIH 1R01NS082561, 1P30NS098577, 5R01CA212148, P50AG05681, P01AG026276, P01AG003991, UL1TR000448, 1P30NS098577, T R01NS103988 and Siemens Healthineers. Avid Radiopharmaceuticals (a wholly owned subsidiary of Eli Lilly) provided the doses of 18F-Florbetapir and partially funded the cost of the PET scans.References
1. Arabi H, Zeng G, Zheng G, Zaidi H. Novel adversarial semantic structure deep learning for MRI-guided attenuation correction in brain PET/MRI. European journal of nuclear medicine and molecular imaging 2019;46(13):2746-2759.2. Blanc-Durand P, Khalife M, Sgard B, Kaushik S, Soret M, Tiss A, El Fakhri G, Habert MO, Wiesinger F, Kas A. Attenuation correction using 3D deep convolutional neural network for brain 18F-FDG PET/MR: Comparison with Atlas, ZTE and CT based attenuation correction. PLoS One 2019;14(10):e0223141.
3. Ladefoged CN, Marner L, Hindsholm A, Law I, Højgaard L, Andersen FL. Deep learning based attenuation correction of PET/MRI in pediatric brain tumor patients: evaluation in a clinical setting. Frontiers in neuroscience 2019;12:1005.
4. Chen Y, Ying C, Binkley MM, Juttukonda MR, Flores S, Laforest R, Benzinger TL, An H. Deep learning‐based T1‐enhanced selection of linear attenuation coefficients (DL‐TESLA) for PET/MR attenuation correction in dementia neuroimaging. Magnetic resonance in medicine 2021;86(1):499-513.
5. Catana C, Laforest R, An HY, Boada F, Cao TY, Faul D, Jakoby B, Jansen FP, Kemp BJ, Kinahan PE, Larson P, Levine MA, Maniawski P, Mawlawi O, McConathy JE, McMillan AB, Price JC, Rajagopal A, Sunderland J, Veit-Haibach P, Wangerin KA, Ying CW, Hope TA. A Path to Qualification of PET/MRI Scanners for Multicenter Brain Imaging Studies: Evaluation of MRI-Based Attenuation Correction Methods Using a Patient Phantom. Journal of Nuclear Medicine 2022;63(4):615-621.
6. Carney JP, Townsend DW, Rappoport V, Bendriem B. Method for transforming CT images for attenuation correction in PET/CT imaging. Med Phys 2006;33(4):976-983.
7. Paulus DH, Quick HH, Geppert C, Fenchel M, Zhan Y, Hermosillo G, Faul D, Boada F, Friedman KP, Koesters T. Whole-body PET/MR imaging: quantitative evaluation of a novel model-based MR attenuation correction method including bone. Journal of Nuclear Medicine 2015;56(7):1061-1066.
Figures
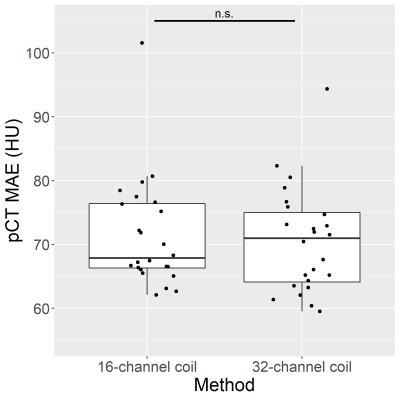
Figure 1. Mean
absolute error (MAE) between CT and pCT using the 16-channel coil and the 32-channel coil.
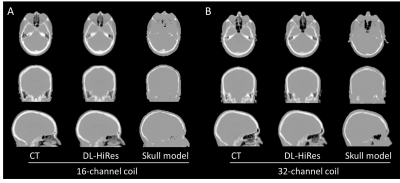
Figure 2. Representative CT μ-map, DL-HiRes pCT μ-map and skull model μ-map using the 16-channel coil (A) and the
32-channel coil (B).
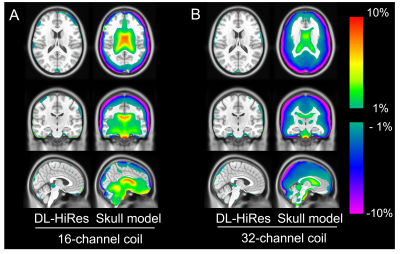
Figure 3. PET mean relative error (MRE) on the
voxel basis across participants in Group A (n = 22; 16-channel coil) and in
Group B (n = 24; 32-channel coil). The acquired CT AC method is used as the
gold standard reference.
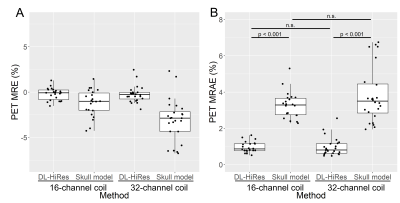
Figure 4. PET mean relative error (MRE;
A) and
mean relative absolute error (MRAE;
B) in
the whole brain. The acquired CT AC method is used as the gold standard
reference.
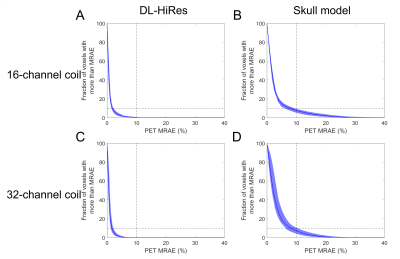
Figure 5. Cumulative voxelwise PET mean relative absolute error (MRAE)
across participants.
The acquired CT AC method is used as the gold standard reference. The solid
blue line and the shaded region show the median and interquartile range across
participants, respectively.
DOI: https://doi.org/10.58530/2023/0892