0886
The validation of ASL-aCBV measured by Hadamard encoded ASL imaging evaluating moyamoya disease correlative study with 15O-H2O PET-aCBV.1Faculty of Medical Sciences, University of Fukui, Eiheiji, Japan, 2Radiology, National Health Insurance Echizen-cho Ota Hospital, Fukui, Japan, 3Neurosurgery, University of Fukui, Eiheiji, Japan, 4Department of Radiological Technolog, Kyoto College of Medical Science, Kyoto, Japan, 5GE Healthcare, Hino, Japan, 6Kumamoto University, Kumamoto, Japan, 7University of Fukui Hospital, Eiheiji, Japan, 8Biomedical Imaging Research Center, University of Fukui, Eiheiji, Japan, 9Department of Radiology, University of Fukui, Eiheiji, Japan
Synopsis
Keywords: Stroke, Perfusion, Arterial spin labeling, CBF, CBV
Positron emission computed tomography (PET) has been used for evaluating cerebral blood flow (CBF) in moyamoya disease to diagnose and assess revascularization result for the patient. Using Hadamard encoded method and DANTE vascular suppression, arterial transit time (ATT) and delay corrected both CBF and aCBV could be obtained in clinical setting. This study aimed to clarify whether ASL- aCBV hemodynamically related to PET-V0 obtained from PET data. There was a significant correlation between PET and CBF both on CBF and aCBV. This may suggest that aCBV could become an additional hemodynamic parameter related to aCBV in completely non-invasive way.Introduction
Cerebral blood flow (CBF) measurement on positron emission computed tomography (PET) has been used to diagnose and assess the treatment for major cerebral artery stenosis/occlusive or moyamoya disease. Cerebral blood volume (CBV) also plays an important role for the maintenance of cerebral blood perfusion. Arterial spin labeling (ASL) also provides a means of non-invasive assessment of CBF on MRI. However, noninvasive measurement of CBV has been challenge because of the difficulty in separating tissue signals from arterial and microvascular signals. The recent paper reported the combination of multi-delay Hadmard ASL acquisition and Delays alternating with nutation for tailored excitation (DANTE) pulses for vascular signal suppression (VS) proposed not only CBF but also arterial cerebral blood volume (aCBV) as an additional hemodynamic parameter [1,2,3]. We hypothesized that this aCBV was also related with the hemodynamic parameters from PET examination for cerebrovascular disease (CVD). Therefore, the objective of this study was to demonstrate the feasibility of quantitative aCBV map in patients with moyamoya disease and to clarify whether aCBV correlates CBV related parameter in PET hemodynamic examination.PET analysis model for CBF and V0
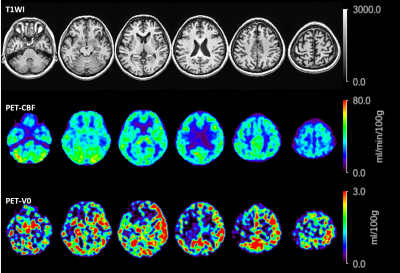
In order to compare PET and ASL on both CBF and CBV, we applied the two compartment-model analysis for PET data. This model has been used to reduce the influence of the cerebral vessels on PET-CBF images and simultaneously to calculating images of cerebral arterial blood volume (V0) [4]. In addition, pixel by pixel tracer delay correction was added for the calculation of PET-V0 map [5].ASL signal model
The used ASL signal model also consists of two compartments corresponding to microvascular and tissue, each has different transit time, i.e., ATT and tissue transit time (TTT), respectively. In addition, we hypothesized that the signal from microvascular compartment could be ideally suppressed by the method using DANTE-VS scheme, while the signal from tissue could be fully retained. We have used the simplified two-compartment model with two transit time consideration, which has been reported previously [3].Materials AND Methods
A whole-body 3.0T PET/MRI scanner (Signa PET/MR, GE Healthcare, Milwaukee, WI, USA) was used for PET data in this study. Eleven patients with moyamoya disease were recruited for this study. The examinations of patients with clinical event were performed within 10 weeks after the onset of symptoms. Institutional review board approval and informed consent were obtained. Nine patients with moyamoya disease (47 ± 14 years old) were scanned on a 3.0 T magnetic resonance imaging unit (Discovery 750, GE Healthcare) with a 32-channel head array coil. H-ASL was performed with LD=40000 ms, PLD=700 ms, three delays, repetition time of 6225 ms, echo time of 10.5 ms, field of view of 240 mm, 512 points with 7 interleaves, and a signal average. Using the data of Hadamard-encoded acquisition, a long-labeled short-delay perfusion image (1dLLSD) was also calculated. In addition, single-delay pCASL with a long LD=4000ms and long PLD=3000 ms was acquired (1dLLLD). We combined two series of ASL acquisition (3d H-pCASL including 1dLLSD and 1dLLLD) to estimate ATTs using the weighted delay method [1]. The combination of 3d and 1dLLLD were repeated again in the same protocol but with VS condition, which make total scan time 10min 42 sec. All calculated maps were spatially normalized to the Montreal Neurological Institute-space template using SPM12 [6]. The volumes of interest in the anterior, middle, and posterior cerebral artery territories were automatically delineated using a vascular territory atlas template [7].Results
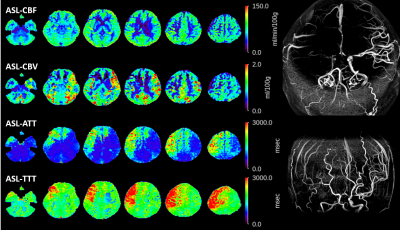
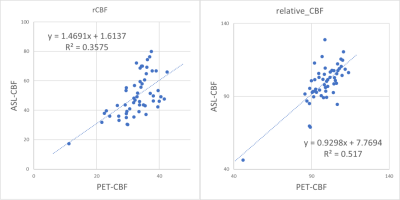
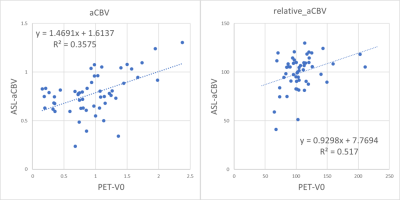
Figure 1 shows a representative case with moyamoya disease. Figure 2 demonstrates the comparison between PET and ASL-CBF in both absolute and relative values. Figure 2 demonstrates the comparison between PET and ASL-aCBV in both absolute and relative value. A significant linear correlation existed between ASL-CBF and PET-CBF (R2=0.36 and 0.52, in absolute and relative value, respectively in all ROIs. A significant linear correlation existed between ASL-aCBV and PET-V0 (R2=0.36 and 0.52, in absolute and relative value, respectively) in all ROIs.Discussion
We have demonstrated the feasibility of simultaneous CBF, ATT and aCBV calculation with the combined usage of Hadamard-ASL and DANTE-VS in clinical setting. There was a significant correlation between PET and ASL both on CBF and aCBV. This may suggest that aCBV could become an additional hemodynamic parameter related to arterial cerebral blood volume in completely non-invasive way.Conclusion
The metrics of aCBV as well as CBF, ATT, TTT based on the ASL signal model may be useful for characterizing the case with misery perfusion state in complete non-invasive way.Acknowledgements
This work was supported in parts by JSPS KAKENHI (grant number 21K15802 and 21K07616).References
1. Dai W, Robson PM, Shankaranarayanan A, Alsop DC. Reduced resolution transit delay prescan for quantitative continuous arterial spin labeling perfusion imaging. Magn Reson Med. 2012;67:1252-1265.
2. Ishida S, Kimura H, Isozaki M, et al. Robust arterial transit time and cerebral blood flow estimation using combined acquisition of Hadamard-encoded multi-delay and long-labeled long-delay pseudo-continuous arterial spin labeling: a simulation and in vivo study. NMR Biomed. 2020;33:e4319.
3. Ishida S, Kimura H, Takei N, et al. Separating spin compartments in arterial spin labeling using delays alternating with nutation for tailored excitation (DANTE) pulse: A validation study using T2 -relaxometry and application to arterial cerebral blood volume imaging. Magn Reson Med. 2022;87:1329-1345.
4. H. Okazawa and M. Vafaee, Effect of vascular radioactivity on regional values of cerebral blood flow: evaluation of methods for H215O PET to distinguish cerebral perfusion from blood volume. Nucl Med 2001 Vol. 42 Issue 7 Pages 1032-9
5. M. M. Islam, T. Tsujikawa, T. Mori, et.al. Pixel-by-pixel precise delay correction for measurement of cerebral hemodynamic parameters in H215O PET study. Ann Nucl Med 2017 Vol. 31 Issue 4 Pages 283-294
6. Ashburner J, Friston KJ. Voxel-based morphometry—the methods. NeuroImage. 2000;11(6 Pt 1):805-821.
7. H. J. Mutsaerts, J. W. van Dalen, D. F. Heijtel, et al., Cerebral Perfusion Measurements in Elderly with Hypertension Using Arterial Spin Labeling. PLoS One 2015 Vol. 10 Issue 8 Pages e0133717
Figures